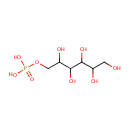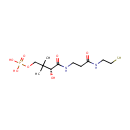
Search Results for compounds
Searching compounds for
returned 4373 results.
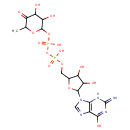
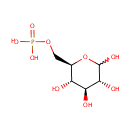
GDP-4-Dehydro-6-L-deoxygalactose (PAMDB000366)
IUPAC:
[({[3,4-dihydroxy-5-(6-hydroxy-2-imino-3,9-dihydro-2H-purin-9-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy][(3,4-dihydroxy-6-methyl-5-oxooxan-2-yl)oxy]phosphinic acid
CAS: Not Available
Description: GDP-4-dehydro-6-L-deoxygalactose is a member of the chemical class known as Purine Nucleotide Sugars. These are purine nucleotides bound to a saccharide derivative through the terminal phosphate group. GDP-6-deoxy-D-talose biosynthetic pathway involve the enzyme GDP-4-keto-6-deoxy-D-mannose-3-dehydratase, which catalyzes the third step in colitose production, which is the removal of the hydroxyl group at C3' of GDP-4-keto-6-deoxymannose. (PMID 16943443)
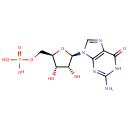
Guanosine monophosphate (PAMDB000369)
IUPAC:
{[(2R,3S,4R,5R)-5-(2-amino-6-oxo-6,9-dihydro-1H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid
CAS: 85-32-5
Description: Guanosine 5'-monophosphate. A guanine nucleotide containing one phosphate group esterified to the sugar moiety and found widely in nature.
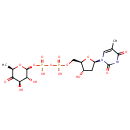
4,6-Dideoxy-4-oxo-dTDP-D-glucose (PAMDB000370)
IUPAC:
{[(3R,4R,6R)-3,4-dihydroxy-6-methyl-5-oxooxan-2-yl]oxy}({[hydroxy({[(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)oxolan-2-yl]methoxy})phosphoryl]oxy})phosphinic acid
CAS: Not Available
Description: 4,6-Dideoxy-4-oxo-dTDP-D-glucose is a product of the enzyme TDP-glucose 4,6-dehydratase [EC:4.2.1.46] in the Nucleotide sugars metabolism (KEGG)
Sorbitol-6-phosphate (PAMDB000371)
IUPAC:
[(2,3,4,5,6-pentahydroxyhexyl)oxy]phosphonic acid
CAS: 20479-58-7
Description: Sorbitol 6-phosphate (Sor6P) is an intermediate in sorbitol biosynthesis. It is a competitive inhibitor for both cytosolic and chloroplastic PGIs with a K(i) of 61 and 40muM, respectively. PMID: 18242768
Glucose 6-phosphate (PAMDB000372)
IUPAC:
{[(2R,3S,4S,5R)-3,4,5,6-tetrahydroxyoxan-2-yl]methoxy}phosphonic acid
CAS: 56-73-5
Description: Glucose 6 phosphate (alpha-D-glucose 6 phosphate or G6P) is the alpha-anomer of glucose-6-phosphate. There are two anomers of glucose 6 phosphate, the alpha anomer and the beta anomer Glucose-6-phosphate is a phosphorylated glucose molecule on carbon 6. When glucose enters a cell, it is immediately phosphorylated to G6P. This is catalyzed with hexokinase enzymes, thus consuming one ATP. A major reason for immediate phosphorylation of the glucose is so that it cannot diffuse out of the cell. The phosphorylation adds a charged group so the G6P cannot easily cross cell membranes. G6P can travel down two metabolic pathways, glycolysis and the pentose phosphate pathway. Note, the molecule now has 2 phosphoryl groups attached. The addition of the 2nd phosphoryl group is an irreversible step, so once this happens G6P will enter glycolysis and be turned into pyruvate (ATP production occurs). After being converted to G6P, phosphoglucose mutase (isomerase) can turn the molecule into glucose-1-phosphate. Glucose-1-phosphate can then be combined with uridine triphosphate (UTP) to form UDP-glucose. This reaction is driven by the hydrolysis of pyrophosphate that is released in the reaction. (Wikipedia)
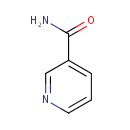
Niacinamide (PAMDB000374)
IUPAC:
pyridine-3-carboxamide
CAS: 98-92-0
Description: Nicotinamide, also known as niacinamide and nicotinic acid amide, is the amide of nicotinic acid (vitamin B3). Nicotinamide is a water-soluble vitamin and is part of the vitamin B group. In cells, niacin is incorporated into nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), although the pathways for nicotinamide and nicotinic acid are very similar. NAD+ and NADP+ are coenzymes in a wide variety of enzymatic oxidation-reduction reactions. (Wikipedia)
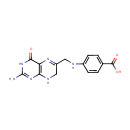
7,8-Dihydropteroic acid (PAMDB000376)
IUPAC:
4-{[(2-amino-4-oxo-3,4,7,8-tetrahydropteridin-6-yl)methyl]amino}benzoic acid
CAS: 2134-76-1
Description: 7,8-dihydropteroate is an intermediate product in dihydrofolate synthesis. It occurs via the enzymic catalysis of the reaction of 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine pyrophosphate with p-aminobenzoate. The enzymes 6-hydroxymethylpterin pyrophosphokinase (EC 2.7.6.3, HPPK) and dihydropteroate synthase (EC 2.5.1.15, DHPS) catalyze sequential steps in folate biosynthesis.
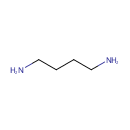
Putrescine (PAMDB000378)
IUPAC:
butane-1,4-diamine
CAS: 110-60-1
Description: Putrescine is a polyamine. Putrescine is related to cadaverine (another polyamine). Both are produced by the breakdown of amino acids in living and dead organisms and both are toxic in large doses. Putrescine and cadaverine are largely responsible for the foul odor of putrefying flesh. Putrescine attacks s-adenosyl methionine and converts it to spermidine. Spermidine in turn attacks another s-adenosyl methionine and converts it to spermine. Putrescine is synthesized in small quantities by healthy living cells by the action of ornithine decarboxylase. The polyamines, of which putrescine is one of the simplest, appear to be growth factors necessary for cell division. (Wikipedia)
Pantetheine 4'-phosphate (PAMDB000379)
IUPAC:
[(3R)-3-hydroxy-2,2-dimethyl-3-({2-[(2-sulfanylethyl)carbamoyl]ethyl}carbamoyl)propoxy]phosphonic acid
CAS: 2226-71-3
Description: Pantetheine 4'-phosphate is a metabolite in the pantothenate and coenzyme A biosynthesis pathway. It can be generated from pantatheine (via pantothenate kinase 1) or R-4'-phospho-pantothenoyl-L-cysteine (via phosphopantothenoylcysteine decarboxylase) or dephospho-CoA (via 4'-phosphopantetheine adenylyl-transferase and ectonucleotide pyrophosphatase). The conversion of pantetheine 4'-phosphate (4'-PP) to dephospho-CoA, is catalyzed by 4'-phosphopantetheine adenylyl-transferase. It has been identified as an essential cofactor in in the biosynthesis of fatty acids, polyketides, depsipeptides, peptides, and compounds derived from both carboxylic and amino acid precursors. In particular it is a key prosthetic group of acyl carrier protein (ACP) and peptidyl carrier proteins (PCP) and aryl carrier proteins (ArCP) derived from Coenzyme A. Phosphopantetheine fulfils two demands. Firstly, the intermediates remain covalently linked to the synthases (or synthetases) in an energy-rich thiol ester linkage. Secondly, the flexibility and length of phosphopantetheine chain (approximately 2 nm) allows the covalently tethered intermediates to have access to spatially distinct enzyme active sites.
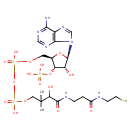
Coenzyme A (PAMDB000381)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-2,2-dimethyl-3-({2-[(2-sulfanylethyl)carbamoyl]ethyl}carbamoyl)propoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 85-61-0
Description: Coenzyme A (CoA, CoASH, or HSCoA) is a coenzyme, notable for its role in the synthesis and oxidization of fatty acids, and the oxidation of pyruvate in the citric acid cycle. It is adapted from beta-mercaptoethylamine, panthothenate and adenosine triphosphate. Acetyl-CoA is an important molecule itself. It is the precursor to HMG CoA, which is a vital component in cholesterol and ketone synthesis. Furthermore, it contributes an acetyl group to choline to produce acetylcholine, in a reaction catalysed by choline acetyltransferase. Its main task is conveying the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. -- Wikipedia