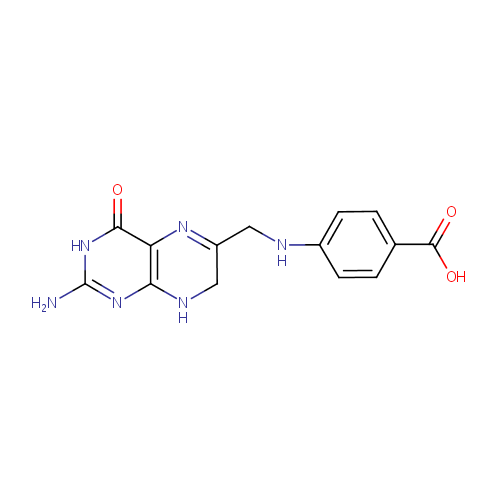
7,8-Dihydropteroic acid (PAMDB000376)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000376 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 7,8-Dihydropteroic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 7,8-dihydropteroate is an intermediate product in dihydrofolate synthesis. It occurs via the enzymic catalysis of the reaction of 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine pyrophosphate with p-aminobenzoate. The enzymes 6-hydroxymethylpterin pyrophosphokinase (EC 2.7.6.3, HPPK) and dihydropteroate synthase (EC 2.5.1.15, DHPS) catalyze sequential steps in folate biosynthesis. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C14H14N6O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 314.2994 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 314.112738344 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | WBFYVDCHGVNRBH-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C14H14N6O3/c15-14-19-11-10(12(21)20-14)18-9(6-17-11)5-16-8-3-1-7(2-4-8)13(22)23/h1-4,16H,5-6H2,(H,22,23)(H4,15,17,19,20,21) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 2134-76-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 4-{[(2-amino-4-oxo-3,4,7,8-tetrahydropteridin-6-yl)methyl]amino}benzoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 7,8-dihydropteroic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC1=NC2=C(N=C(CNC3=CC=C(C=C3)C(O)=O)CN2)C(=O)N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pterins and derivatives. These are polycyclic aromatic compounds containing a pterin moiety, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pteridines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pterins and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pterins and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | p-Aminobenzoic acid + 6-Hydroxymethyl-dihydropterin pyrophosphate > 7,8-Dihydropteroic acid + Pyrophosphate Adenosine triphosphate + 7,8-Dihydropteroic acid + L-Glutamate <> ADP + Phosphate + Dihydrofolic acid 6-Hydroxymethyl dihydropterin + p-Aminobenzoic acid <> 7,8-Dihydropteroic acid + Water L-Glutamate + 7,8-Dihydropteroic acid + Adenosine triphosphate > Hydrogen ion + Dihydrofolic acid + Phosphate + ADP Adenosine triphosphate + 7,8-Dihydropteroic acid + L-Glutamate > ADP + Inorganic phosphate + Dihydrofolic acid 6-Hydroxymethyl-dihydropterin pyrophosphate + p-Aminobenzoic acid > Pyrophosphate + 7,8-Dihydropteroic acid 7,8-Dihydropteroic acid + Adenosine triphosphate + L-Glutamic acid + L-Glutamate > Adenosine diphosphate + Phosphate + Hydrogen ion + 7,8-dihydrofolate monoglutamate + ADP + Dihydrofolic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Bartels, Rainer; Bock, Lothar. Determination of pteroic acid by high-performance thin-layer chromatography. Contribution to the investigation of 7,8-dihydropteroate synthase. Journal of Chromatography (1994), 659(1), 185-9. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||