Search Results for compounds
Searching compounds for
returned 4373 results.
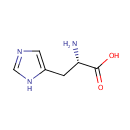
L-Histidine (PAMDB000074)
IUPAC:
(2S)-2-amino-3-(1H-imidazol-5-yl)propanoic acid
CAS: 71-00-1
Description: Histidine is an amino acid. It is a precursor for histamine and carnosine biosynthesis. The enzyme histidine ammonia-lyase converts histidine into ammonia and urocanic acid. (Wikipedia)
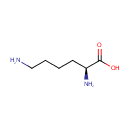
L-Lysine (PAMDB000075)
IUPAC:
(2S)-2,6-diaminohexanoic acid
CAS: 56-87-1
Description: L-lysine is an alpha-amino acid with the chemical formula HO2CCH(NH2)(CH2)4NH2. Lysine is a basic amino acid as are arginine and histidine. Lysine is a proteogenic amino acid, meaning that it is used in protein synthesis. Lysine typically constitutes about 7-8% of an average protein. The epsilon-amino group often participates in hydrogen bonding and as a general base in catalysis. Common posttranslational modifications include methylation of the epsilon-amino group, giving methyl-, dimethyl-, and trimethyllysine. In bacteria, lysine is synthesized from aspartic acid, which is first converted to aspartyl-semialdehyde.
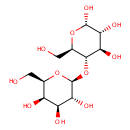
alpha-Lactose (PAMDB000076)
IUPAC:
(2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-{[(2R,3S,4R,5R,6S)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}oxane-3,4,5-triol
CAS: 63-42-3
Description: Alpha-Lactose is the alpha anomoer of lactose. It is a disaccharide that consists of galactose and glucose joined by an acetal oxygen bridge in the beta orientation Lactose is a sugar substrate that can be readily used by Pseudomonas aeruginosa. The consumption of lactose by Pseudomonas aeruginosa is controlled by the lac operon. The operon includes beta-galactosidase, lactose permease, and thiogalactoside transacetylase. The lactose permease, which sits in the cytoplasmic membrane, transports lactose into the cell. Beta-galactosidase, a cytoplasmic enzyme, subsequently cleaves lactose into glucose and galactose.
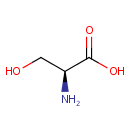
L-Serine (PAMDB000077)
IUPAC:
(2S)-2-amino-3-hydroxypropanoic acid
CAS: 56-45-1
Description: Serine is an amino acid derived from glycine. Like all the amino acid building blocks of protein and peptides, serine can become essential under certain conditions. Serine is highly concentrated in all cell membranes. (http://www.dcnutrition.com/AminoAcids/) L-Serine may be derived from biosynthesis from the glycolytic intermediate 3-phosphoglycerate; from glycine ; and by protein and phospholipid degradation. Little data is available on the relative contributions of each of these sources of l-serine to serine homoeostasis. In the biosynthetic pathway, the glycolytic intermediate 3-phosphoglycerate is converted into phosphohydroxypyruvate, in a reaction catalyzed by 3-phosphoglycerate dehydrogenase (3- PGDH; EC 1.1.1.95). Phosphohydroxypyruvate is metabolized to phosphoserine by phosphohydroxypyruvate aminotransferase (EC 2.6.1.52) and, finally, phosphoserine is converted into l-serine by phosphoserine phosphatase (PSP; EC 3.1.3.3). Of the three synthetic enzymes, the properties of 3-PGDH and PSP are the best documented. L-Serine is the predominant source of one-carbon groups for the de novo synthesis of purine nucleotides and deoxythymidine monophosphate. It has long been recognized that, in cell cultures, L-serine is a conditional essential amino acid, because it cannot be synthesized in sufficient quantities to meet the cellular demands for its utilization.
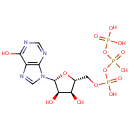
Inosine triphosphate (PAMDB000078)
IUPAC:
({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(6-hydroxy-9H-purin-9-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid
CAS: 132-06-9
Description: Inosine triphosphate (ITP) is an intermediate in the purine metabolism pathway. ITPase is a cytosolic nucleoside triphosphate pyrophosphohydrolase specific for ITP catalysis to inosine monophosphate (IMP) and deoxy-inosine triphosphate (dITP) to deoxy-inosine monophosphate. ITPase's function is not clearly understood but possible roles for ITPase could be to prevent the accumulation of rogue nucleotides which would be otherwise incorporated into DNA and RNA, or compete with nucleotides such as GTP in signalling processes. (PMID : 170291, 1204209, 17113761, 17924837)
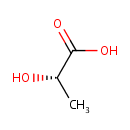
L-Lactic acid (PAMDB000079)
IUPAC:
(2S)-2-hydroxypropanoic acid
CAS: 79-33-4
Description: Lactic acid plays a role in several biochemical processes. Pseudomonas aeruginosa produces lactic acid from pyruvate in a process of normal metabolism. The lactic acid produced by Pseudomonas aeruginosa and other probiotics (mostly lactic acid bacteria) can lower the pH in mammalian host intestines and therefore, it inhibits the growth of proteolytic bacteria and have a beneficial effect on intestinal inflammation. (PMID 15138208)
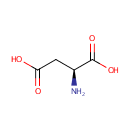
L-Aspartic acid (PAMDB000080)
IUPAC:
(2S)-2-aminobutanedioic acid
CAS: 56-84-8
Description: Aspartic acid (Asp, D), also known as aspartate (the name of its anion), is one of the 20 natural proteinogenic amino acids which are the building blocks of proteins. Aspartic acid has 2 enantiomeric forms: D and L. L-aspartic acid is the isomer with more biological roles and is the form used in constructing proteins. (Wikipedia) In Pseudomonas aeruginosa, L-aspartate can be produced from L-glutamate, and interconversion can occur between L-asparagine and L-aspartate. L-aspartate is also involved in the biosynthesis pathways of many compounds, including adenosine nucleotides, beta-alanine, and homoserine. (EcoCyc)
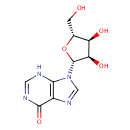
Inosine (PAMDB000082)
IUPAC:
9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6,9-dihydro-3H-purin-6-one
CAS: 58-63-9
Description: Inosine is purine nucleoside that has hypoxanthine linked by the N9 nitrogen to the C1 carbon of ribose. It is an intermediate in the degradation of purines and purine nucleosides to uric acid and in pathways of purine salvage. It also occurs in the anticodon of certain transfer RNA molecules. (Dorland, 28th ed)
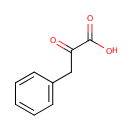
Phenylpyruvic acid (PAMDB000084)
IUPAC:
2-oxo-3-phenylpropanoic acid
CAS: 156-06-9
Description: Phenylpyruvic acid is a keto-acid that is an intermediate or catabolic byproduct of phenylalanine metabolism. It has a slight honey-like odor. Phenylalanine is converted to phenylpyruvic acid. In particular, excessive phenylalanine can be metabolized into phenylketones through, a transaminase pathway route involving glutamate. Metabolites of this transamination reaction include phenylacetate, phenylpyruvate and phenethylamine.
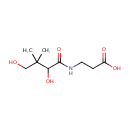
Pantothenic acid (PAMDB000085)
IUPAC:
3-(2,4-dihydroxy-3,3-dimethylbutanamido)propanoic acid
CAS: 79-83-4
Description: Pantothenic acid, also called vitamin B5, is a water-soluble vitamin required to sustain life. Pantothenic acid is needed to form coenzyme-A (CoA), and is thus critical in the metabolism and synthesis of carbohydrates, proteins, and fats. Its name is derived from the Greek pantothen meaning "from everywhere" and small quantities of pantothenic acid are found in nearly every food, with high amounts in whole grain cereals, legumes, eggs, meat, and royal jelly.