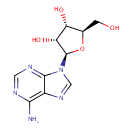
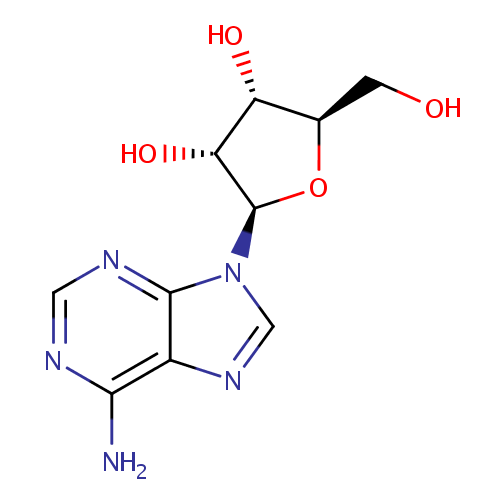
| References: |
- Ballantyne PJ: Social context and outcomes for the ageing breast cancer patient: considerations for clinical practitioners. J Clin Nurs. 2004 Mar;13(3a):11-21. Pubmed: 15028034
- Bennett, B. D., Kimball, E. H., Gao, M., Osterhout, R., Van Dien, S. J., Rabinowitz, J. D. (2009). "Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli." Nat Chem Biol 5:593-599. Pubmed: 19561621
- Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD: The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005 Sep 22;437(7058):574-8. Pubmed: 16177794
- Dunne VG, Bhattachayya S, Besser M, Rae C, Griffin JL: Metabolites from cerebrospinal fluid in aneurysmal subarachnoid haemorrhage correlate with vasospasm and clinical outcome: a pattern-recognition 1H NMR study. NMR Biomed. 2005 Feb;18(1):24-33. Pubmed: 15455468
- Eells JT, Spector R: Purine and pyrimidine base and nucleoside concentrations in human cerebrospinal fluid and plasma. Neurochem Res. 1983 Nov;8(11):1451-7. Pubmed: 6656991
- Gheorghiade M, Teerlink JR, Mebazaa A: Pharmacology of new agents for acute heart failure syndromes. Am J Cardiol. 2005 Sep 19;96(6A):68G-73G. Pubmed: 16181825
- Ishii, N., Nakahigashi, K., Baba, T., Robert, M., Soga, T., Kanai, A., Hirasawa, T., Naba, M., Hirai, K., Hoque, A., Ho, P. Y., Kakazu, Y., Sugawara, K., Igarashi, S., Harada, S., Masuda, T., Sugiyama, N., Togashi, T., Hasegawa, M., Takai, Y., Yugi, K., Arakawa, K., Iwata, N., Toya, Y., Nakayama, Y., Nishioka, T., Shimizu, K., Mori, H., Tomita, M. (2007). "Multiple high-throughput analyses monitor the response of E. coli to perturbations." Science 316:593-597. Pubmed: 17379776
- Jansen RW, Kruijt JK, van Berkel TJ, Meijer DK: Coupling of the antiviral drug ara-AMP to lactosaminated albumin leads to specific uptake in rat and human hepatocytes. Hepatology. 1993 Jul;18(1):146-52. Pubmed: 7686877
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Koeris M, Funke L, Shrestha J, Rich A, Maas S: Modulation of ADAR1 editing activity by Z-RNA in vitro. Nucleic Acids Res. 2005 Sep 21;33(16):5362-70. Print 2005. Pubmed: 16177183
- Lee SH, Jung BH, Kim SY, Chung BC: A rapid and sensitive method for quantitation of nucleosides in human urine using liquid chromatography/mass spectrometry with direct urine injection. Rapid Commun Mass Spectrom. 2004;18(9):973-7. Pubmed: 15116424
- Maytin M, Colucci WS: Cardioprotection: a new paradigm in the management of acute heart failure syndromes. Am J Cardiol. 2005 Sep 19;96(6A):26G-31G. Pubmed: 16181820
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2(1):18. Pubmed: 15882454
- Skalhegg BS, Funderud A, Henanger HH, Hafte TT, Larsen AC, Kvissel AK, Eikvar S, Orstavik S: Protein kinase A (PKA)--a potential target for therapeutic intervention of dysfunctional immune cells. Curr Drug Targets. 2005 Sep;6(6):655-64. Pubmed: 16178799
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Vidotto C, Fousert D, Akkermann M, Griesmacher A, Muller MM: Purine and pyrimidine metabolites in children's urine. Clin Chim Acta. 2003 Sep;335(1-2):27-32. Pubmed: 12927681
- Vijayendran, C., Barsch, A., Friehs, K., Niehaus, K., Becker, A., Flaschel, E. (2008). "Perceiving molecular evolution processes in Escherichia coli by comprehensive metabolite and gene expression profiling." Genome Biol 9:R72. Pubmed: 18402659
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
- Yamamoto T, Moriwaki Y, Takahashi S, Fujita T, Tsutsumi Z, Yamakita J, Shimizu K, Shiota M, Ohta S, Higashino K: Determination of adenosine and deoxyadenosine in urine by high-performance liquid chromatography with column switching. J Chromatogr B Biomed Sci Appl. 1998 Nov 20;719(1-2):55-61. Pubmed: 9869364
|
|---|