|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001755 |
|---|
|
Identification |
|---|
| Name: |
Sulfoacetate |
|---|
| Description: | Sulfoacetate is a member of the chemical class known as Sulfonic Acids. These are compounds containing the sulfonic acid group, which has the general structure RS(=O)2OH (R = H). Sulfoacetate is invovled in Taurine and hypotaurine metabolism. (KEGG) |
|---|
|
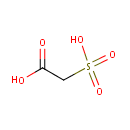
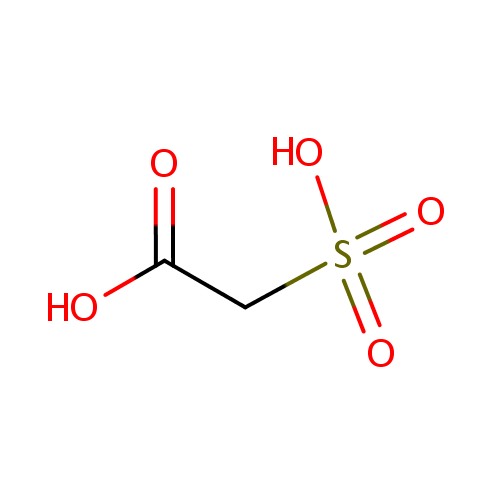
Structure |
|
|---|
| Synonyms: | - 2-Sulfoacetate
- 2-Sulfoacetic acid
- 2-Sulphoacetate
- 2-Sulphoacetic acid
- Sulfoacetate
- Sulfoacetic acid
- Sulfoethanoate
- Sulfoethanoic acid
- Sulphoacetate
- Sulphoacetic acid
- Sulphoethanoate
- Sulphoethanoic acid
|
|---|
|
Chemical Formula: |
C2H4O5S |
|---|
| Average Molecular Weight: |
140.115 |
|---|
| Monoisotopic Molecular
Weight: |
139.977943928 |
|---|
| InChI Key: |
AGGIJOLULBJGTQ-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C2H4O5S/c3-2(4)1-8(5,6)7/h1H2,(H,3,4)(H,5,6,7) |
|---|
| CAS
number: |
123-43-3 |
|---|
| IUPAC Name: | 2-sulfoacetic acid |
|---|
|
Traditional IUPAC Name: |
sulfoacetic acid |
|---|
| SMILES: | OC(=O)CS(O)(=O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as sulfonic acids. These are compounds containing the sulfonic acid group, which has the general structure RS(=O)2OH (R is not a hydrogen atom). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Sulfonic acids and derivatives |
|---|
| Sub Class | Sulfonic acids |
|---|
|
Direct Parent |
Sulfonic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alkanesulfonic acid
- Sulfonyl
- Sulfonic acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
85 °C |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|