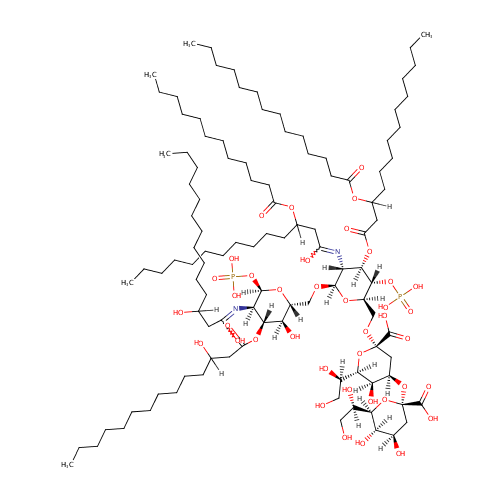
KDO2-Lipid A (PAMDB001447)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001447 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | KDO2-Lipid A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | KDO2-Lipid A is an intermediate in the synthesis of LPS. It has two 3-deoxy-D-manno-octulosonic acid (KDO) sugar residues in place of the core, and has no O-antigen. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C110H202N2O39P2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 2238.7184 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 2237.335997756 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | DIXUKJUHGLIZGU-ZKUMOPDFSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C110H202N2O39P2/c1-7-13-19-25-31-37-38-44-50-56-62-68-92(123)142-82(66-60-54-48-42-35-29-23-17-11-5)72-94(125)146-104-96(112-90(121)71-81(65-59-53-47-41-34-28-22-16-10-4)141-91(122)67-61-55-49-43-36-30-24-18-12-6)105(144-88(102(104)150-152(133,134)135)78-140-109(107(129)130)74-86(98(127)101(148-109)85(119)76-114)147-110(108(131)132)73-83(117)97(126)100(149-110)84(118)75-113)139-77-87-99(128)103(145-93(124)70-80(116)64-58-52-46-40-33-27-21-15-9-3)95(106(143-87)151-153(136,137)138)111-89(120)69-79(115)63-57-51-45-39-32-26-20-14-8-2/h79-88,95-106,113-119,126-128H,7-78H2,1-6H3,(H,111,120)(H,112,121)(H,129,130)(H,131,132)(H2,133,134,135)(H2,136,137,138)/t79?,80?,81?,82?,83-,84-,85-,86-,87-,88-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,109-,110-/m1/s1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2R,4R,5R,6R)-4-{[(2R,4R,5R,6R)-2-carboxy-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-[(1,3-dihydroxytetradecylidene)amino]-3-hydroxy-4-[(3-hydroxytetradecanoyl)oxy]-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}-5-hydroxyoxane-2-carboxylic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (2R,4R,5R,6R)-4-{[(2R,4R,5R,6R)-2-carboxy-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-[(1,3-dihydroxytetradecylidene)amino]-3-hydroxy-4-[(3-hydroxytetradecanoyl)oxy]-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}-5-hydroxyoxane-2-carboxylic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H]C(O)(CCCCCCCCCCC)CC(=O)O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO[C@]2([H])O[C@]([H])(CO[C@@]3(C[C@@]([H])(O[C@@]4(C[C@@]([H])(O)[C@@]([H])(O)[C@]([H])(O4)[C@]([H])(O)CO)C(O)=O)[C@@]([H])(O)[C@]([H])(O3)[C@]([H])(O)CO)C(O)=O)[C@@]([H])(OP(O)(O)=O)[C@]([H])(OC(=O)CC([H])(CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@]2([H])N=C(O)CC([H])(CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)O[C@]([H])(OP(O)(O)=O)[C@]1([H])N=C(O)CC([H])(O)CCCCCCCCCCC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as hexacarboxylic acids and derivatives. These are carboxylic acids containing exactly six carboxyl groups. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Hexacarboxylic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Hexacarboxylic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Water + KDO2-Lipid A > ADP + Hydrogen ion + Phosphate + KDO2-Lipid A Adenosine triphosphate + Water + KDO2-Lipid A > ADP + Hydrogen ion + Phosphate + KDO2-Lipid A -->-->KDO(2)-lipid IV(A) with laurate + Myristoyl-ACP (n-C14:0ACP) > acyl carrier protein + KDO2-Lipid A KDO2-Lipid A + PE(14:0/14:0) > 1,2-Diacyl-sn-glycerol (dihexadec-9-enoyl, n-C16:1) + Phosphoethanolamine KDO(2)-lipid (A) KDO2-Lipid A + PE(14:0/14:0) > 1,2-Diacyl-sn-glycerol (dioctadec-11-enoyl, n-C18:1) + Phosphoethanolamine KDO(2)-lipid (A) ADP-L-Glycero-D-manno-heptose + KDO2-Lipid A > ADP + Hydrogen ion + heptosyl-kdo2-lipidA KDO2-Lipid A + Acyl-carrier protein + Tetradecanoyl-[acp] + Lauroyl-KDO2-lipid IV(A) <> Tetradecanoyl-[acp] + Lauroyl-KDO2-lipid IV(A) + Acyl-carrier protein KDO2-Lipid A + 4-amino-4-deoxy-α-L-arabinopyranosyl <i>ditrans,octacis</i>-undecaprenyl phosphate > L-Ara4N-modified KDO2-Lipid A + Di-trans,poly-cis-undecaprenyl phosphate Hydrogen ion + KDO2-Lipid A + ADP-L-Glycero-D-manno-heptose > Heptosyl-KDO2-lipid A + ADP undecaprenyl phosphate-4-amino-4-deoxy-L-arabinose + KDO2-Lipid A + 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate <> alpha-Kdo-(2->4)-alpha-Kdo-(2->6)-[4-P-L-Ara4N]-lipid A + Di-trans,poly-cis-undecaprenyl phosphate + Lipid IIA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in transferase activity, transferring pentosyl groups
- Specific function:
- Catalyzes the transfer of the L-Ara4N moiety of the glycolipid undecaprenyl phosphate-alpha-L-Ara4N to lipid A. The modified arabinose is attached to lipid A and is required for resistance to polymyxin and cationic antimicrobial peptides
- Gene Name:
- arnT
- Locus Tag:
- PA3556
- Molecular weight:
- 61.7 kDa
Reactions
| 4-amino-4-deoxy-alpha-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphate + lipid IV(A) = lipid II(A) + di-trans,octa-cis-undecaprenyl phosphate. |
- General function:
- Involved in transferase activity, transferring glycosyl groups
- Specific function:
- Heptose transfer to the lipopolysaccharide core. It transfers the innnermost heptose to [4'-P](3-deoxy-D-manno- octulosonic acid)2-IVA
- Gene Name:
- rfaC
- Locus Tag:
- PA5011
- Molecular weight:
- 39.7 kDa
- General function:
- Involved in lipopolysaccharide transport
- Specific function:
- Part of the ABC transporter complex lptBFG involved in the translocation of lipopolysaccharide (LPS) from the inner membrane to the outer membrane
- Gene Name:
- lptG
- Locus Tag:
- PA3827
- Molecular weight:
- 39.2 kDa
- General function:
- Involved in lipopolysaccharide-transporting ATPase acti
- Specific function:
- Part of the ABC transporter complex lptBFG involved in the translocation of lipopolysaccharide (LPS) from the inner membrane to the outer membrane
- Gene Name:
- lptF
- Locus Tag:
- PA3828
- Molecular weight:
- 41.3 kDa
- General function:
- Involved in nucleotide binding
- Specific function:
- Involved in lipid A export and possibly also in glycerophospholipid export and for biogenesis of the outer membrane. Transmembrane domains (TMD) form a pore in the inner membrane and the ATP-binding domain (NBD) is responsible for energy generation
- Gene Name:
- msbA
- Locus Tag:
- PA4997
- Molecular weight:
- 66.4 kDa
- General function:
- Involved in nucleotide binding
- Specific function:
- Part of the ABC transporter complex lptBFG involved in the translocation of lipopolysaccharide (LPS) from the inner membrane to the outer membrane. Probably responsible for energy coupling to the transport system
- Gene Name:
- lptB
- Locus Tag:
- PA4461
- Molecular weight:
- 26.5 kDa
- General function:
- Involved in lipopolysaccharide transmembrane transporter activity
- Specific function:
- Required for the translocation of lipopolysaccharide (LPS) from the inner membrane to the outer membrane
- Gene Name:
- lptC
- Locus Tag:
- PA4459
- Molecular weight:
- 21.4 kDa
- General function:
- Involved in lipopolysaccharide binding
- Specific function:
- Required for the translocation of lipopolysaccharide (LPS) from the inner membrane to the outer membrane. May act as a chaperone that facilitates LPS transfer across the aquaeous environment of the periplasm. Interacts specifically with the lipid A domain of LPS
- Gene Name:
- lptA
- Locus Tag:
- PA0005
- Molecular weight:
- 28.7 kDa
Transporters
- General function:
- Involved in lipopolysaccharide transport
- Specific function:
- Part of the ABC transporter complex lptBFG involved in the translocation of lipopolysaccharide (LPS) from the inner membrane to the outer membrane
- Gene Name:
- lptG
- Locus Tag:
- PA3827
- Molecular weight:
- 39.2 kDa
- General function:
- Involved in lipopolysaccharide-transporting ATPase acti
- Specific function:
- Part of the ABC transporter complex lptBFG involved in the translocation of lipopolysaccharide (LPS) from the inner membrane to the outer membrane
- Gene Name:
- lptF
- Locus Tag:
- PA3828
- Molecular weight:
- 41.3 kDa
- General function:
- Involved in nucleotide binding
- Specific function:
- Involved in lipid A export and possibly also in glycerophospholipid export and for biogenesis of the outer membrane. Transmembrane domains (TMD) form a pore in the inner membrane and the ATP-binding domain (NBD) is responsible for energy generation
- Gene Name:
- msbA
- Locus Tag:
- PA4997
- Molecular weight:
- 66.4 kDa