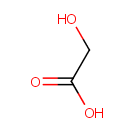
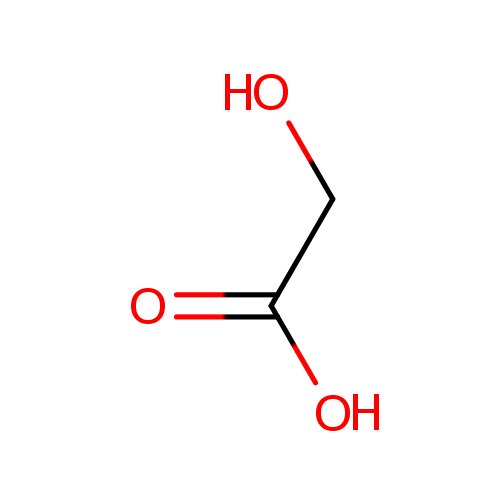
Glycolic acid (PAMDB000475)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000475 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Glycolic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Glycolic acid (or hydroxyacetic acid) is the smallest alpha-hydroxy acid (AHA). In its pure form, glycolic acid is a colorless crystalline solid. Due to its excellent capability to penetrate skin, glycolic acid finds applications in skin care products, most often as a chemical peel. Glycolic acid is also used for tattoo removal. In Pseudomonas aeruginosa it is involved in glyoxylate and dicarboxylate metabolism. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C2H4O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 76.0514 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 76.016043994 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | AEMRFAOFKBGASW-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C2H4O3/c3-1-2(4)5/h3H,1H2,(H,4,5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 79-14-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-hydroxyacetic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | glycolic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OCC(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as alpha hydroxy acids and derivatives. These are organic compounds containing a carboxylic acid substituted with a hydroxyl group on the adjacent carbon. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Hydroxy acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Alpha hydroxy acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Alpha hydroxy acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 75-80 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Glyoxylic acid + Hydrogen ion + NADPH + Glycolate <> Glycolic acid + NADP Glycolic acid + Ubiquinone-8 > Glyoxylic acid + Ubiquinol-8 Glycolic acid + Menaquinone 8 > Glyoxylic acid + Menaquinol 8 2-Demethylmenaquinone 8 + Glycolic acid > 2-Demethylmenaquinol 8 + Glyoxylic acid Glyoxylic acid + Hydrogen ion + NADH > Glycolic acid + NAD Glycolaldehyde + Water + NAD > Glycolic acid +2 Hydrogen ion + NADH Phosphoglycolic acid + Water <> Glycolic acid + Phosphate + Glycolate Glycolic acid + NADP <> Glyoxylic acid + NADPH + Hydrogen ion Glycolic acid + Oxygen <> Glyoxylic acid + Hydrogen peroxide Phosphoglycolic acid + Water <> Glycolic acid + Phosphate an oxidized electron acceptor + Glycolic acid > a reduced electron acceptor + Glyoxylic acid Glycolaldehyde + NAD + Water > Glycolic acid + NADH Glycolic acid + NADP > Glyoxylic acid + NADPH Phosphoglycolic acid + Water > Glycolic acid + Inorganic phosphate Glycolic acid + an oxidized electron-transfer flavoprotein? > Reduced acceptor + Glyoxylic acid 2 Glycolic acid + 2 an oxidized electron-transfer flavoprotein? >2 Reduced acceptor +2 Glyoxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Witzemann, Edgar J. Preparation of glycollic acid. Journal of the American Chemical Society (1917), 39 109-12. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor
- Specific function:
- Catalyzes the NADPH-dependent reduction of glyoxylate and hydroxypyruvate into glycolate and glycerate, respectively. Can also reduce 2,5-diketo-D-gluconate (25DKG) to 5-keto-D- gluconate (5KDG), 2-keto-D-gluconate (2KDG) to D-gluconate, and 2- keto-L-gulonate (2KLG) to L-idonate (IA), but it is not its physiological function. Inactive towards 2-oxoglutarate, oxaloacetate, pyruvate, 5-keto-D-gluconate, D-fructose and L- sorbose. Activity with NAD is very low
- Gene Name:
- ghrB
- Locus Tag:
- PA2263
- Molecular weight:
- 35.6 kDa
Reactions
| Glycolate + NADP(+) = glyoxylate + NADPH. |
| D-glycerate + NAD(P)(+) = hydroxypyruvate + NAD(P)H. |
| D-gluconate + NADP(+) = 2-dehydro-D-gluconate + NADPH. |
- General function:
- Involved in catalytic activity
- Specific function:
- Specific function unknown
- Gene Name:
- glcD
- Locus Tag:
- PA5355
- Molecular weight:
- 53.7 kDa
- General function:
- Involved in iron-sulfur cluster binding
- Specific function:
- Specific function unknown
- Gene Name:
- glcF
- Locus Tag:
- PA5353
- Molecular weight:
- 44.7 kDa
- General function:
- Involved in catalytic activity
- Specific function:
- Specific function unknown
- Gene Name:
- glcE
- Locus Tag:
- PA5354
- Molecular weight:
- 38.2 kDa
Transporters
- General function:
- Involved in lactate transmembrane transporter activity
- Specific function:
- Transports L-lactate across the membrane. Can also transport D-lactate and glycolate. Seems to be driven by a proton motive force
- Gene Name:
- lldP
- Locus Tag:
- PA4770
- Molecular weight:
- 58.7 kDa
- General function:
- Involved in transporter activity
- Specific function:
- Transports acetate. Also able to transport glycolate
- Gene Name:
- actP
- Locus Tag:
- PA3234
- Molecular weight:
- 58.7 kDa