Riboflavin (PAMDB000103)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000103 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
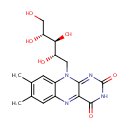
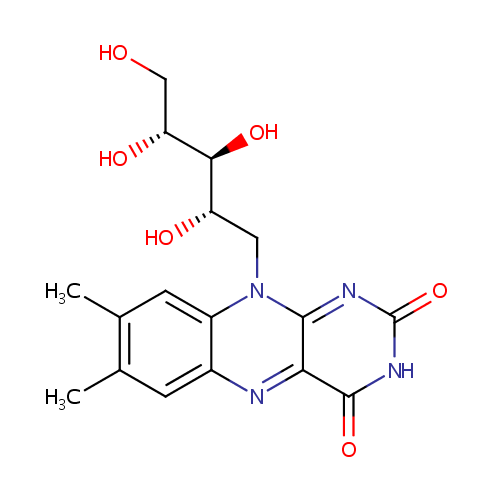
| Name: | Riboflavin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Riboflavin, also known as vitamin B2, is the central component of the cofactors FAD and FMN, and is therefore required by all flavoproteins. As such, vitamin B2 is required for a wide variety of cellular processes. Like the other B vitamins, it plays a key role in energy metabolism, and is required for the metabolism of fats, ketone bodies, carbohydrates, and proteins. (Wikipedia) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C17H20N4O6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 376.3639 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 376.138284392 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | AUNGANRZJHBGPY-SCRDCRAPSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C17H20N4O6/c1-7-3-9-10(4-8(7)2)21(5-11(23)14(25)12(24)6-22)15-13(18-9)16(26)20-17(27)19-15/h3-4,11-12,14,22-25H,5-6H2,1-2H3,(H,20,26,27)/t11-,12+,14-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 83-88-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 7,8-dimethyl-10-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]-2H,3H,4H,10H-benzo[g]pteridine-2,4-dione | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | riboflavin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=C(C)C=C2N(C[C@H](O)[C@H](O)[C@H](O)CO)C3=NC(=O)NC(=O)C3=NC2=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as flavins. These are compounds containing a flavin (7,8-dimethyl-benzo[g]pteridine-2,4-dione) moiety, with a structure characterized by an isoalloaxzine tricyclic ring. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pteridines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Alloxazines and isoalloxazines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Flavins | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 290 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Hydrogen ion + NADPH + Riboflavin > NADP + Reduced riboflavin Adenosine triphosphate + Riboflavin <> ADP + Flavin Mononucleotide + Hydrogen ion Hydrogen ion + NADH + Riboflavin > NAD + Reduced riboflavin 2 Ferroxamine + Reduced riboflavin >2 Iron +2 ferroxamine minus Fe(3) +2 Hydrogen ion + Riboflavin 2 6,7-Dimethyl-8-(1-D-ribityl)lumazine <> Riboflavin + 5-Amino-6-ribitylamino uracil Adenosine triphosphate + Riboflavin <> ADP + Flavin Mononucleotide Hydrogen ion + 6,7-Dimethyl-8-(1-D-ribityl)lumazine > 5-amino-6-(D-ribitylamino)uracil + Riboflavin Reduced riboflavin + NAD(P)<sup>+</sup> Riboflavin + NAD(P)H + Hydrogen ion Flavin Mononucleotide + Water > Riboflavin + Phosphate Reduced riboflavin + NAD(P)(+) > Riboflavin + NAD(P)H | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Tishler, Max; Pfister, Karl, III; Babson, R. D.; Ladenburg, Kurt; Fleming, Ann J. Reaction between o-aminoazo compounds and barbituric acid. A new synthesis of riboflavin. Journal of the American Chemical Society (1947), 69 1487-92. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in acid phosphatase activity
- Specific function:
- Dephosphorylates several organic phosphomonoesters and catalyzes the transfer of low-energy phosphate groups from phosphomonoesters to hydroxyl groups of various organic compounds. Preferentially acts on aryl phosphoesters. Might function as a broad-spectrum dephosphorylating enzyme able to scavenge both 3'- and 5'-nucleotides and also additional organic phosphomonoesters
- Gene Name:
- aphA
- Locus Tag:
- PA1409
- Molecular weight:
- 38 kDa
Reactions
| A phosphate monoester + H(2)O = an alcohol + phosphate. |
- General function:
- Involved in riboflavin synthase activity
- Specific function:
- Riboflavin synthase is a bifunctional enzyme complex catalyzing the formation of riboflavin from 5-amino-6-(1'-D)- ribityl-amino-2,4(1H,3H)-pyrimidinedione and L-3,4-dihydrohy-2- butanone-4-phosphate via 6,7-dimethyl-8-lumazine. The alpha subunit catalyzes the dismutation of 6,7-dimethyl-8-lumazine to riboflavin and 5-amino-6-(1'-D)-ribityl-amino-2,4(1H,3H)- pyrimidinedione
- Gene Name:
- ribE
- Locus Tag:
- PA4053
- Molecular weight:
- 16.4 kDa
Reactions
| 2 6,7-dimethyl-8-(1-D-ribityl)lumazine = riboflavin + 4-(1-D-ribitylamino)-5-amino-2,6-dihydroxypyrimidine. |
- General function:
- Involved in FMN adenylyltransferase activity
- Specific function:
- ATP + riboflavin = ADP + FMN
- Gene Name:
- ribF
- Locus Tag:
- PA4561
- Molecular weight:
- 34.3 kDa
Reactions
| ATP + riboflavin = ADP + FMN. |
| ATP + FMN = diphosphate + FAD. |
- General function:
- Involved in sulfite reductase (NADPH) activity
- Specific function:
- Component of the sulfite reductase complex that catalyzes the 6-electron reduction of sulfite to sulfide. This is one of several activities required for the biosynthesis of L- cysteine from sulfate
- Gene Name:
- cysI
- Locus Tag:
- PA1838
- Molecular weight:
- 62.1 kDa
Reactions
| H(2)S + 3 NADP(+) + 3 H(2)O = sulfite + 3 NADPH. |