|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001686 |
|---|
|
Identification |
|---|
| Name: |
Reduced riboflavin |
|---|
| Description: | Reduced riboflavin is a member of the chemical class known as Quinoxalines. These are compounds containing a quinoxaline moiety, a bicyclic heterocycle made up of a benzene ring fused to a pyrazine ring. Compared to riboflavin, reduced riboflavin has one extra hydrogen attached to an oxygen on its ring structure. Riboflavin, also known as vitamin B2, is the central component of the cofactors FAD and FMN, and is therefore required by all flavoproteins. As such, vitamin B2 is required for a wide variety of cellular processes. It plays a key role in energy metabolism, and for the metabolism of fats, ketone bodies, carbohydrates, and proteins. The reduced form, which occurs in metabolism along with the oxidized form, is colorless. (Wikipedia) |
|---|
|
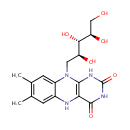
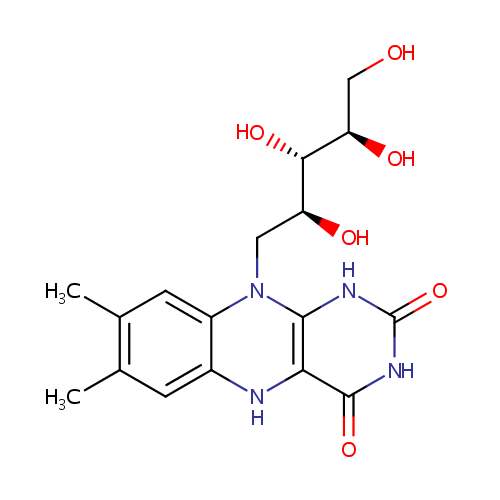
Structure |
|
|---|
| Synonyms: | - 1-Deoxy-1-(7,8-dimethyl-2,4-dioxo-1,3,4,5-tetrahydrobenzo[g]pteridin-10(2H)-yl)-D-ribitol
- 4a,5-Dihydroriboflavine
- 7,8-Dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)-4a,5-dihydroisoalloxazine
- 7,8-Dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)-5,10-dihydrobenzo[g]pteridine-2,4(3H,4aH)-dione
- Reduced riboflavin
|
|---|
|
Chemical Formula: |
C15H16N4O6 |
|---|
| Average Molecular Weight: |
348.3107 |
|---|
| Monoisotopic Molecular
Weight: |
348.106984264 |
|---|
| InChI Key: |
ATANIONNQLTUND-CKYFFXLPSA-N |
|---|
| InChI: | InChI=1S/C15H16N4O6/c20-6-10(22)12(23)9(21)5-19-8-4-2-1-3-7(8)16-11-13(19)17-15(25)18-14(11)24/h1-4,9-10,12,20-23H,5-6H2,(H,18,24,25)/t9-,10-,12-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 7,8-dimethyl-10-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]-1H,2H,3H,4H,5H,10H-benzo[g]pteridine-2,4-dione |
|---|
|
Traditional IUPAC Name: |
reduced riboflavin |
|---|
| SMILES: | OC[C@@H](O)[C@H](O)[C@H](O)CN1C2=CC=CC=C2N=C2C(=O)NC(=O)N=C12 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as flavins. These are compounds containing a flavin (7,8-dimethyl-benzo[g]pteridine-2,4-dione) moiety, with a structure characterized by an isoalloaxzine tricyclic ring. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Pteridines and derivatives |
|---|
| Sub Class | Alloxazines and isoalloxazines |
|---|
|
Direct Parent |
Flavins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Flavin
- Alkyldiarylamine
- Pyrimidone
- Benzenoid
- Pyrimidine
- Heteroaromatic compound
- Vinylogous amide
- Urea
- Tertiary amine
- Secondary alcohol
- Polyol
- Lactam
- 1,2-diol
- Azacycle
- Secondary amine
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|