|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000944 |
|---|
|
Identification |
|---|
| Name: |
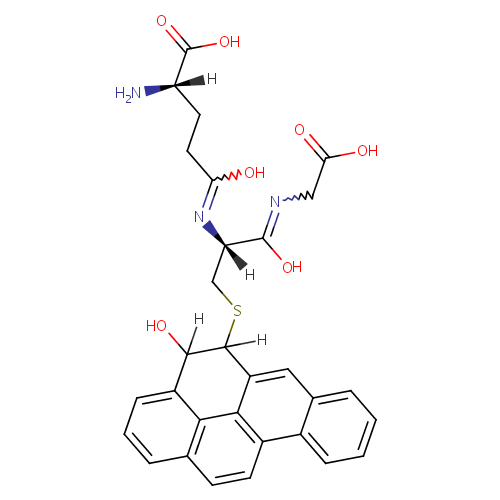
4,5-Dihydro-4-hydroxy-5-S-glutathionyl-benzo[a]pyrene |
|---|
| Description: | 4,5-dihydro-4-hydroxy-5-s-glutathionyl-benzo[a]pyrene is a member of the chemical class known as Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. 3-amino-2-propanol is invovled in L-threonine metabolism. D-1-Amino-2-propanol:NAD+ oxidoreductase activity, which catalyzes the second step in a pathway wherein L-threonine is converted to D-1-amino-2-propanol via the intermediate formation of aminoacetone, has been purified 500-fold from Pseudomonas aeruginosa. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (R)-1-aminopropan-2-ol
- 1-Amino-2-propanol
- 3-Amino-2-propanol
- Aminopropanol
- D-1-Amino-2-propanol
- D-1-Aminopropan-2-ol
- DL-1-amino-2-propanol
|
|---|
|
Chemical Formula: |
C30H29N3O7S |
|---|
| Average Molecular Weight: |
575.632 |
|---|
| Monoisotopic Molecular
Weight: |
575.172620987 |
|---|
| InChI Key: |
PZXCOGFLSNREMF-SUZCJKPRSA-N |
|---|
| InChI: | InChI=1S/C30H29N3O7S/c31-21(30(39)40)10-11-23(34)33-22(29(38)32-13-24(35)36)14-41-28-20-12-16-4-1-2-6-17(16)18-9-8-15-5-3-7-19(27(28)37)25(15)26(18)20/h1-9,12,21-22,27-28,37H,10-11,13-14,31H2,(H,32,38)(H,33,34)(H,35,36)(H,39,40)/t21-,22-,27?,28?/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-({11-hydroxypentacyclo[10.6.2.0?,??0?????0??????icosa-1(19),2,4,6,8,12,14,16(20),17-nonaen-10-yl}sulfanyl)ethyl]-C-hydroxycarbonimidoyl}butanoic acid |
|---|
|
Traditional IUPAC Name: |
(2S)-2-amino-4-{[(1R)-1-(carboxymethyl-C-hydroxycarbonimidoyl)-2-({11-hydroxypentacyclo[10.6.2.0?,??0?????0??????icosa-1(19),2,4,6,8,12,14,16(20),17-nonaen-10-yl}sulfanyl)ethyl]-C-hydroxycarbonimidoyl}butanoic acid |
|---|
| SMILES: | [H][C@](N)(CCC(O)=N[C@@]([H])(CSC1([H])C2=CC3=CC=CC=C3C3=C2C2=C(C=CC=C2C1([H])O)C=C3)C(O)=NCC(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
Peptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha peptide
- Chrysene
- Phenanthrene
- N-acyl-aliphatic-alpha amino acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- D-alpha-amino acid
- Naphthalene
- Alpha-amino acid or derivatives
- Alpha-amino acid
- Amino fatty acid
- Fatty acyl
- Benzenoid
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Dialkylthioether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Sulfenyl compound
- Thioether
- Carboxylic acid
- Carboximidic acid derivative
- Carboximidic acid
- Hydrocarbon derivative
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Alcohol
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aromatic homopolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Campbell, R. L., Swain, R. R., Dekker, E. E. (1978). "Purification, separation, and characterization of two molecular forms of D-1-amino-2-propanol:NAD+ oxidoreductase activity from extracts of Escherichia coli K-12." J Biol Chem 253:7282-7288. Pubmed: 359547
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB60391 | | Pubchem Compound ID | 11954068 | | Kegg ID | C14855 | | ChemSpider ID | 10128363 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|