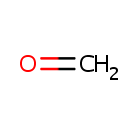Formaldehyde (PAMDB000382)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000382 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Formaldehyde | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Formaldehyde is a highly reactive aldehyde gas. It is the simplest aldehyde. Its chemical formula is H2CO. Although formaldehyde is a gas at room temperature, it is readily soluble in water, and it is most commonly sold as a 37% solution in water called by trade names such as formalin or formol. In water, formaldehyde polymerizes, and formalin actually contains very little formaldehyde in the form of H2CO monomer. Formaldehyde exhibits most of the general chemical properties of the aldehydes, except that is generally more reactive than other aldehydes. Formaldehyde is a potent electrophile. It can participate in electrophilic aromatic substitution reactions with aromatic compounds and can undergo electrophilic addition reactions with alkenes. In Pseudomonas aeruginosa, formaldehyde is formed by the breakdown of N-methyltryptophan and FMNH by their corresponding oxidases. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | CH2O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 30.026 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 30.010564686 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | WSFSSNUMVMOOMR-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/CH2O/c1-2/h1H2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 50-00-0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | formaldehyde | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | formaldehyde | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C=O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as hydrocarbon derivatives. These are derivatives of hydrocarbons obtained by substituting one or more carbon atoms by an heteroatom. THey contain at least one carbon atom and heteroatom. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Hydrocarbon derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Hydrocarbon derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | -92 °C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | FMNH + Methanesulfonate + Oxygen > Formaldehyde + Flavin Mononucleotide + Hydrogen ion + Water + Sulfite Water + Oxygen + Sarcosine > Formaldehyde + Glycine + Hydrogen peroxide N-Methyltryptophan + Water + Oxygen > Formaldehyde + Hydrogen peroxide + L-Tryptophan Formaldehyde + Glutathione <> S-(Hydroxymethyl)glutathione Methanol + Hydrogen peroxide <> Formaldehyde +2 Water Formaldehyde + Tetrahydrofolic acid > 5,10-Methylene-THF + Water a methylated nucleobase within DNA + Oxygen + Oxoglutaric acid Hydrogen ion + a nucleobase within DNA + Carbon dioxide + Formaldehyde + Succinic acid N1-Methyladenine + Oxygen + Oxoglutaric acid > Hydrogen ion + Adenine + Carbon dioxide + Formaldehyde + Succinic acid N3-Methylcytosine + Oxygen + Oxoglutaric acid > Hydrogen ion + Cytosine + Carbon dioxide + Formaldehyde + Succinic acid DNA-base-CH(3) + Oxoglutaric acid + Oxygen > DNA-base + Formaldehyde + Succinic acid + Carbon dioxide | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalase activity
- Specific function:
- Decomposes hydrogen peroxide into water and oxygen; serves to protect cells from the toxic effects of hydrogen peroxide
- Gene Name:
- katE
- Locus Tag:
- PA2147
- Molecular weight:
- 78 kDa
Reactions
| 2 H(2)O(2) = O(2) + 2 H(2)O. |
- General function:
- Involved in FMN reductase activity
- Specific function:
- Catalyzes an NAD(P)H-dependent reduction of FMN, but is also able to reduce FAD or riboflavin
- Gene Name:
- ssuE
- Locus Tag:
- PA3446
- Molecular weight:
- 21.5 kDa
Reactions
| FMNH(2) + NADP(+) = FMN + NADPH. |
- General function:
- Involved in alkanesulfonate monooxygenase activity
- Specific function:
- Involved in desulfonation of aliphatic sulfonates. Catalyzes the conversion of pentanesulfonic acid to sulfite and pentaldehyde and is able to desulfonate a wide range of sulfonated substrates including C-2 to C-10 unsubstituted linear alkanesulfonates, substituted ethanesulfonic acids and sulfonated buffers
- Gene Name:
- ssuD
- Locus Tag:
- PA3444
- Molecular weight:
- 41.6 kDa
Reactions
| An alkanesufonate (R-CH(2)-SO(3)H) + FMNH(2) + O(2) = an aldehyde (R-CHO) + FMN + sulfite + H(2)O. |