|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000177 |
|---|
|
Identification |
|---|
| Name: |
Isovaleric acid |
|---|
| Description: | Isovaleric acid, is a natural short-chain fatty acid. It is clear colorless liquid that is sparingly soluble in water, but extremely soluble in most common organic solvents. |
|---|
|
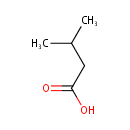
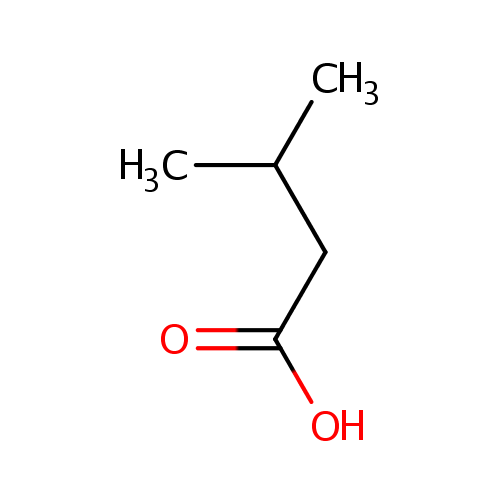
Structure |
|
|---|
| Synonyms: | - 3-Methyl-N-butyrate
- 3-Methyl-N-butyric acid
- 3-Methylbutanoate
- 3-Methylbutanoic acid
- 3-Methylbutyrate
- 3-Methylbutyrate: isopropyl-acetate
- 3-Methylbutyrate: isopropyl-Acetic acid
- 3-Methylbutyric acid
- 3-Methylbutyric acid: isopropyl-Acetate
- 3-Methylbutyric acid: isopropyl-Acetic acid
- B-Methylbutyrate
- B-Methylbutyric acid
- Beta-Methylbutyrate
- Beta-Methylbutyric acid
- Delphinate
- Delphinic acid
- Isopentanoate
- Isopentanoic acid
- Isopropylacetate
- Isopropylacetic acid
- Isovalerate
- Isovalerianate
- Isovalerianic acid
- Isovaleric acid
- β-Methylbutyrate
- β-Methylbutyric acid
|
|---|
|
Chemical Formula: |
C5H10O2 |
|---|
| Average Molecular Weight: |
102.1317 |
|---|
| Monoisotopic Molecular
Weight: |
102.068079564 |
|---|
| InChI Key: |
GWYFCOCPABKNJV-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C5H10O2/c1-4(2)3-5(6)7/h4H,3H2,1-2H3,(H,6,7) |
|---|
| CAS
number: |
503-74-2 |
|---|
| IUPAC Name: | 3-methylbutanoic acid |
|---|
|
Traditional IUPAC Name: |
isovaleric acid |
|---|
| SMILES: | CC(C)CC(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as methyl-branched fatty acids. These are fatty acids with an acyl chain that has a methyl branch. Usually, they are saturated and contain only one or more methyl group. However, branches other than methyl may be present. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
|
Direct Parent |
Methyl-branched fatty acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Methyl-branched fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
-29.3 °C |
|---|
| Experimental Properties: |
| Property | Value | Source |
|---|
| Water Solubility: | 40.7 mg/ml [YALKOWSKY,SH & DANNENFELSER,RM (1992)] | PhysProp | | LogP: | 1.039 | PhysProp |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0gvo-9300000000-16b5d15d26810b72bb36 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0006-9000000000-692791435c37fa74a692 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0006-9000000000-b4c1b59653cd4d803637 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7M) , Positive | splash10-03dl-9000000000-b0f7e399edb7f95572ed | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80B) , Positive | splash10-03dl-9000000000-c8be68873f336c8de6d6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0udi-0900000000-d0f20bc9813dcba79e8b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0udi-0900000000-63b09019c280c88ee64d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0udi-1900000000-ef9ee29c1febd59e6a00 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9200000000-e825f0aa9ab54c1695d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9000000000-9ba126f982634fdc9406 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-afb863a56c8763c30251 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zfr-8900000000-244535e4070d536a29b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pc0-9300000000-93ed2bc326380cb0d880 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-44b2fa11a5a6daab8dd0 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-03dl-9000000000-e7c77b2bc7f3ac5191e9 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Ackman RG: Birthweights in the Faroe Islands: possible role of isovaleric acid. J Intern Med. 1989 Feb;225(2):73-5. Pubmed: 2646392
- Ara K, Hama M, Akiba S, Koike K, Okisaka K, Hagura T, Kamiya T, Tomita F: Foot odor due to microbial metabolism and its control. Can J Microbiol. 2006 Apr;52(4):357-64. Pubmed: 16699586
- Arthur K, Hommes FA: Simple isotope dilution assay for propionic acid and isovaleric acid. J Chromatogr B Biomed Appl. 1995 Nov 3;673(1):132-5. Pubmed: 8925066
- Hoffmann GF, von Kries R, Klose D, Lindner M, Schulze A, Muntau AC, Roschinger W, Liebl B, Mayatepek E, Roscher AA: Frequencies of inherited organic acidurias and disorders of mitochondrial fatty acid transport and oxidation in Germany. Eur J Pediatr. 2004 Feb;163(2):76-80. Epub 2004 Jan 9. Pubmed: 14714182
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Silva MF, Selhorst J, Overmars H, van Gennip AH, Maya M, Wanders RJ, de Almeida IT, Duran M: Characterization of plasma acylcarnitines in patients under valproate monotherapy using ESI-MS/MS. Clin Biochem. 2001 Nov;34(8):635-8. Pubmed: 11849623
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: |
Imada, Yukio; Mizuno, Sumiko; Mikawa, Takashi. Manufacture of isovaleric acid and 2-methylbutyric acid. Jpn. Kokai Tokkyo Koho (1986), 3 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|