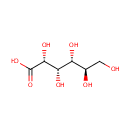
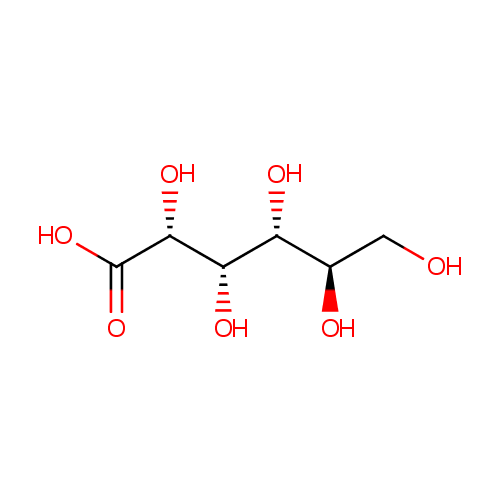
Gluconic acid (PAMDB000160)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000160 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Gluconic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Gluconic acid occurs naturally in fruit, honey and wine and is used as a food additive, an acidity regulator. It is also used in cleaning products where it helps cleaning up mineral deposits. It is a strong chelating agent, especially in alkaline solution. It chelates the anions of calcium, iron, aluminium, copper, and other heavy metals. Glucono delta lactone is a cyclic ester of D-gluconic acid. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H12O7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 196.1553 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 196.058302738 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | RGHNJXZEOKUKBD-SQOUGZDYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H12O7/c7-1-2(8)3(9)4(10)5(11)6(12)13/h2-5,7-11H,1H2,(H,12,13)/t2-,3-,4+,5-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 526-95-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | gluconate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as sugar acids and derivatives. These are compounds containing a saccharide unit which bears a carboxylic acid group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Sugar acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Sugar acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 113-118 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Ubiquinone-8 + D-Glucose + Water > Ubiquinol-8 + Gluconic acid + Hydrogen ion Adenosine triphosphate + Gluconic acid <> 6-Phosphogluconic acid + ADP + Hydrogen ion 2 -Dehydro-L-gulonate + Hydrogen ion + NADH > Gluconic acid + NAD 2 -Dehydro-L-gulonate + Hydrogen ion + NADPH > Gluconic acid + NADP 5-Keto-D-gluconate + Hydrogen ion + NADPH <> Gluconic acid + NADP Adenosine triphosphate + Gluconic acid <> ADP + 6-Phosphogluconic acid Gluconic acid + NADP <> 2-Keto-D-gluconic acid + NADPH + Hydrogen ion + 2-Dehydro-D-gluconate NADP + Gluconic acid <> Hydrogen ion + 2-Dehydro-D-gluconate + NADPH NAD(P)<sup>+</sup> + Gluconic acid <> NAD(P)H + 5-Keto-D-gluconate + Hydrogen ion Gluconolactone + Water > Hydrogen ion + Gluconic acid 6-Phosphogluconic acid + Water > Gluconic acid + Phosphate Gluconic acid + NADP > 2-Dehydro-D-gluconate + NADPH Gluconic acid + NAD(P)(+) > 5-Keto-D-gluconate + NAD(P)H Gluconic acid + NAD + NADP <> 5-Keto-D-gluconate + NADH + NADPH + Hydrogen ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Anastassiadis, Savas; Morgunov, Igor G. Gluconic acid production. Recent Patents on Biotechnology (2007), 1(2), 167-180. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in oxidoreductase activity, acting on CH-OH group of donors
- Specific function:
- GDH is probably involved in energy conservation rather than in sugar metabolism
- Gene Name:
- gcd
- Locus Tag:
- PA2290
- Molecular weight:
- 86.2 kDa
Reactions
| D-glucose + ubiquinone = D-glucono-1,5-lactone + ubiquinol. |
- General function:
- Involved in oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor
- Specific function:
- Catalyzes the NADPH-dependent reduction of glyoxylate and hydroxypyruvate into glycolate and glycerate, respectively. Can also reduce 2,5-diketo-D-gluconate (25DKG) to 5-keto-D- gluconate (5KDG), 2-keto-D-gluconate (2KDG) to D-gluconate, and 2- keto-L-gulonate (2KLG) to L-idonate (IA), but it is not its physiological function. Inactive towards 2-oxoglutarate, oxaloacetate, pyruvate, 5-keto-D-gluconate, D-fructose and L- sorbose. Activity with NAD is very low
- Gene Name:
- ghrB
- Locus Tag:
- PA2263
- Molecular weight:
- 35.6 kDa
Reactions
| Glycolate + NADP(+) = glyoxylate + NADPH. |
| D-glycerate + NAD(P)(+) = hydroxypyruvate + NAD(P)H. |
| D-gluconate + NADP(+) = 2-dehydro-D-gluconate + NADPH. |
- General function:
- Involved in oxidoreductase activity, acting on the CH-OH group of donors, quinone or similar compound as acceptor
- Specific function:
- Aldose sugar dehydrogenase with broad substrate specificity. The physiological substrate is unknown. Can oxidize glucose to gluconolactone. Can also utilize D-arabinose, L- arabinose and 2-deoxy-glucose. Has higher activity towards oligomeric sugars, such as maltose, maltotriose or cellobiose. It may function to input sugar-derived electrons into the respiratory network
- Gene Name:
- yliI
- Locus Tag:
- PA1112
- Molecular weight:
- 41.4 kDa