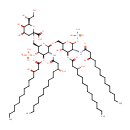
Search Results for compounds
Searching compounds for
returned 4373 results.
Hexadecanoyl-phosphate (n-C16:0) (PAMDB001634)
IUPAC:
(hexadecanoyloxy)phosphonate
CAS: Not Available
Description: Hexadecanoyl-phosphate (n-c16:0) belongs to the class of Acyl Phosphates. These are organic compounds containing the functional group -CO-P(O)(O)OH. (inferred from compound structure)
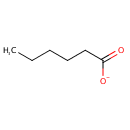
Hexanoate (N-C6:0) (PAMDB001637)
IUPAC:
hexanoate
CAS: Not Available
Description: Hexanoate (n-c6:0) belongs to the class of Carboxylic Acid Salts. These are ionic derivatives of carboxylic acid. (inferred from compound structure)Hexanoic acid (caproic acid), is the carboxylic acid derived from hexane with the general formula C5H11COOH. It is a colorless oily liquid with an odor that is fatty, cheesy, waxy, and like that of goats or other barnyard animals. It is a fatty acid found naturally in various animal fats and oils, and is one of the chemicals that give the decomposing fleshy seed coat of the ginkgo its characteristic unpleasant odor. The primary use of hexanoic acid is in the manufacture of its esters for artificial flavors, and in the manufacture of hexyl derivatives such as hexylphenols. The salts and esters of this acid are known as hexanoates or caproates. Two other acids are named after goats: caprylic (C8) and capric (C10). Along with hexanoic acid, these total 15% in goat milk fat. Caproic, caprylic, and capric acids (capric is a crystal- or wax-like substance, whereas the other 2 are mobile liquids) are not only used for the formation of esters but also commonly used neat in: butter, milk, cream, strawberry, bread, beer, nut, and other flavors. (WikiPedia)
Hydrogen cyanide (PAMDB001639)
IUPAC:
formonitrile
CAS: 74-90-8
Description: HCN is formed in interstellar clouds through one of two major pathways: via a neutral-neutral reaction (CH2 + N <=> HCN + H) and via dissociative recombination (HCNH+ + e- <=> HCN + H). The dissociative recombination pathway is dominant by 30%; however, the HCNH+ must be in its linear form. Dissociative recombination with its structural isomer, H2NC+ produces hydrogen isocyanide (HNC), exclusively.; HCN is produced on an industrial scale and is a highly valuable precursor to many chemical compounds ranging from polymers to pharmaceuticals.; Hydrogen cyanide (with the historical common name of Prussic acid) is an inorganic compound with chemical formula HCN. It is a colorless, extremely poisonous liquid that boils slightly above room temperature at 26
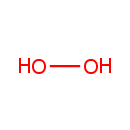
Hydrogen peroxide (PAMDB001640)
IUPAC:
peroxol
CAS: 7722-84-1
Description: Hydrogen peroxide (H2O2) is the simplest peroxide (a compound with an oxygen-oxygen single bond). It is also a strong oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. The oxidizing capacity of hydrogen peroxide is so strong that it is considered a highly reactive oxygen species. Organisms also naturally produce hydrogen peroxide as a by-product of oxidative metabolism. Consequently, nearly all living things (specifically, all obligate and facultative aerobes) possess enzymes known as catalase peroxidases, which harmlessly and catalytically decompose low concentrations of hydrogen peroxide to water and oxygen. (Wikipedia) Hydrogen peroxide (H2O2) is a very pale blue liquid which appears colourless in a dilute solution, slightly more viscous than water. It is a weak acid. It has strong oxidizing properties and has also found use as a disinfectant and as an oxidizer. Hydrogen peroxide (H2O2) is a well-documented component of living cells. It plays important roles in host defense and oxidative biosynthetic reactions. In addition, there is growing evidence that at low levels, H2O2 also functions as a signaling agent, particularly in higher organisms. (HMDB) In Pseudomonas aeruginosa, H2O2 is used in the aerobic degradation of L-ascorbate, and produced as a byproduct in many reactions involving oxygen as an oxidizing agent. (EcoCyc) In Pseudomonas aeruginosa glyoxylate metabolism, H2O2 is produced as a byproduct of the conversion of glycolate to glyoxylate by the enzyme glycolate oxidase (EC 1.1.3.15), and H2O2 is then converted to harmless O2 by the enzyme catalase (EC 1.11.1.6). (KEGG)
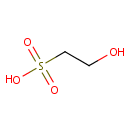
Isethionic acid (PAMDB001641)
IUPAC:
2-hydroxyethane-1-sulfonic acid
CAS: 107-36-8
Description: Isethionic acid (C2H6O4S) is a short chain alkane sulfonate containing hydroxy group, is a water soluble liquid used in the manufacture of mild, biodegradable and high foaming anionic surfactants which provides gentle cleansing and soft skin feel.; A colorless, syrupy, strongly acidic liquid that can form detergents with oleic acid. The Pseudomonas aeruginosa ssuEADCB gene cluster is required for the utilization of alkanesulfonates (such as isethionic acid) as sulfur sources, and is expressed under conditions of sulfate or cysteine starvation.
KDO(2)-lipid IV(A) (PAMDB001643)
IUPAC:
(2R,4R,5R,6R)-4-{[(2R,4R,5R,6R)-2-carboxy-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-5-hydroxy-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-3-hydroxy-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-3-(phosphonooxy)oxan-2-yl]methoxy}oxane-2-carboxylic acid
CAS: 143600-83-3
Description: Kdo(2)-lipid iv(a) belongs to the class of Polysaccharide Phosphates. These are polysaccharides in which a phosphate group is bound to at least one carbohydrate unit. (inferred from compound structure)
KDO-lipid IV(A) (PAMDB001644)
IUPAC:
(2R,4R,5R,6R)-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxy-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-3-hydroxy-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-3-(phosphonooxy)oxan-2-yl]methoxy}oxane-2-carboxylic acid
CAS: Not Available
Description: KDO-lipid IV(a) is a part of lipopolysaccharide or LPS. Specifically it is lipid IVA glycosylated with a single 3-deoxy-D-manno-octulosonic acid (KDO) residue. KDO-lipid IV(a) is a saccharolipid. The most familiar saccharolipids are the acylated glucosamine precursors of the lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many as seven fatty-acyl chains. The minimal lipopolysaccharide required for growth in Pseudomonas aeruginosa is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.
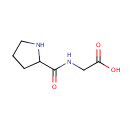
L-Prolinylglycine (PAMDB001645)
IUPAC:
2-(pyrrolidin-2-ylformamido)acetic acid
CAS: 2578-57-6
Description: Prolinylglycine is a dipeptide. It is a proteolytic breakdown product of larger proteins. Pseudomonas aeruginosa can use this dipeptide as a substrate for carbon/nitrogen metabolism and a source of proline. It is transported via DppA, which appears to be both a dipeptide transporter and a dipeptidase. Pro-Gly utilization does not require any of the three major proline transport systems. [PMID:1702779]
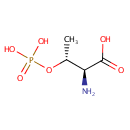
L-Threonine O-3-phosphate (PAMDB001646)
IUPAC:
(2S,3R)-2-amino-3-(phosphonooxy)butanoic acid
CAS: 1114-81-4
Description: In Pseudomonas aeruginosa, acid phosphatase / phosphotransferase and alkaline phosphatase are the enzymes that catalyze the chemical reaction L-threonine 3-O-phosphate[periplasmic space] + H2O[periplasmic space] -> L-threonine[periplasmic space] + phosphate[periplasmic space], where L-Threonine O-3-phosphate is a substrate (EcoCyc compound: L-THREONINE-O-3-PHOSPHATE).
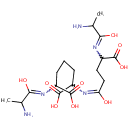
L-Alanine-D-glutamate-meso-2,6-diaminoheptanedioate-D-alanine (PAMDB001647)
IUPAC:
2-[(2-amino-1-hydroxypropylidene)amino]-6-({4-[(2-amino-1-hydroxypropylidene)amino]-4-carboxy-1-hydroxybutylidene}amino)heptanedioic acid
CAS: Not Available
Description: L-alanine-d-glutamate-meso-2,6-diaminoheptanedioate-d-alanine belongs to the class of Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. (inferred from compound structure)