|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120559 |
|---|
|
Identification |
|---|
| Name: |
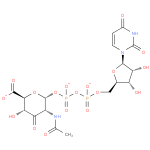
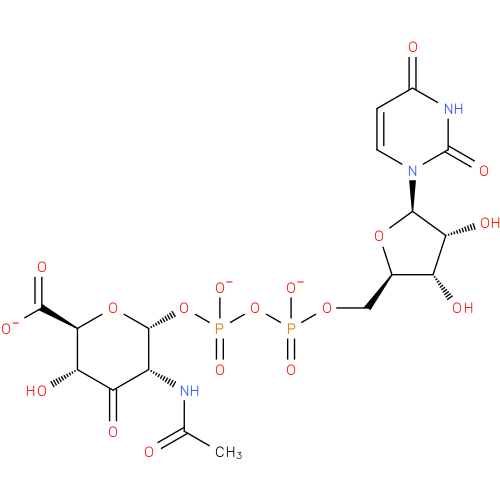
UDP-2-acetamido-2-deoxy-α-D-ribo-hex-3-uluronate |
|---|
| Description: | A nucleotide-sugar oxoanion obtained via deprotonation of the diphosphate and carboxy OH groups of UDP-2-acetamido-2-deoxy-α-D-ribo-hex-3-uluronic acid; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | - UDP-2-acetamido-2-deoxy-α-D-ribo-hex-3-uluronate
- UDP-3-keto-α-D-GlcNAcA (3−)
- UDP-α-GlcNAc(3keto)A(3−)
- UDP-GlcNAc(3keto)A(3−)
|
|---|
|
Chemical Formula: |
C17H20N3O18P2 |
|---|
| Average Molecular Weight: |
616.302 |
|---|
| Monoisotopic Molecular
Weight: |
619.04517 |
|---|
| InChI Key: |
FQYJGWJSECSVLP-AZKAKUJRSA-K |
|---|
| InChI: | InChI=1S/C17H23N3O18P2/c1-5(21)18-8-10(24)11(25)13(15(27)28)36-16(8)37-40(32,33)38-39(30,31)34-4-6-9(23)12(26)14(35-6)20-3-2-7(22)19-17(20)29/h2-3,6,8-9,11-14,16,23,25-26H,4H2,1H3,(H,18,21)(H,27,28)(H,30,31)(H,32,33)(H,19,22,29)/p-3/t6-,8-,9-,11+,12-,13+,14-,16-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | Not Available |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC(=O)NC1(C(C(C(C(=O)[O-])OC1OP([O-])(=O)OP([O-])(OCC3(OC(N2(C(NC(=O)C=C2)=O))C(O)C3O))=O)O)=O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine nucleotide sugars |
|---|
|
Direct Parent |
Pyrimidine nucleotide sugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine nucleotide sugar
- Pyrimidine ribonucleoside diphosphate
- Pentose-5-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- Organic pyrophosphate
- Monosaccharide phosphate
- Pyrimidone
- Beta-hydroxy acid
- Organic phosphoric acid derivative
- Pyran
- Oxane
- Alkyl phosphate
- Hydroxy acid
- Phosphoric acid ester
- Pyrimidine
- Hydropyrimidine
- Monosaccharide
- Acetamide
- Vinylogous amide
- Heteroaromatic compound
- Tetrahydrofuran
- Carboxamide group
- Ketone
- Lactam
- Cyclic ketone
- Urea
- Secondary carboxylic acid amide
- Secondary alcohol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Organic anion
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- UDP-2,3-diacetamido-2,3-dideoxy-α-D-mannuronate biosynthesisPWY-7090
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Thoden JB, Holden HM (2010)Structural and functional studies of WlbA: A dehydrogenase involved in the biosynthesis of 2,3-diacetamido-2,3-dideoxy-D-mannuronic acid . Biochemistry 49, Pubmed: 20690587
- Larkin A, Olivier NB, Imperiali B (2010)Structural analysis of WbpE from Pseudomonas aeruginosa PAO1: a nucleotide sugar aminotransferase involved in O-antigen assembly. Biochemistry 49, Pubmed: 20604544
- Westman EL, Preston A, Field RA, Lam JS (2008)Biosynthesis of a rare di-N-acetylated sugar in the lipopolysaccharides of both Pseudomonas aeruginosa and Bordetella pertussis occurs via an identical scheme despite different gene clusters. Journal of bacteriology 190, Pubmed: 18621892
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|