|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120551 |
|---|
|
Identification |
|---|
| Name: |
baicalein |
|---|
| Description: | A trihydroxyflavone with the hydroxy groups at positions C-5, -6 and -7. |
|---|
|
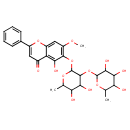
Structure |
|
|---|
| Synonyms: | - 5,6,7-Trihydroxyflavone
- Baicalein
- baicalein (OLD)
|
|---|
|
Chemical Formula: |
C15H10O5 |
|---|
| Average Molecular Weight: |
270.241 |
|---|
| Monoisotopic Molecular
Weight: |
270.05283 |
|---|
| InChI Key: |
FXNFHKRTJBSTCS-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H |
|---|
| CAS
number: |
192224-98-9 |
|---|
| IUPAC Name: | 5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one |
|---|
|
Traditional IUPAC Name: |
6-({4,5-dihydroxy-6-methyl-3-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl}oxy)-5-hydroxy-7-methoxy-2-phenylchromen-4-one |
|---|
| SMILES: | C1(C=CC(=CC=1)C2(=CC(=O)C3(=C(O2)C=C(O)C(O)=C(O)3))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as flavonoid o-glycosides. These are compounds containing a carbohydrate moiety which is O-glycosidically linked to the 2-phenylchromen-4-one flavonoid backbone. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
|
Class |
Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
|
Direct Parent |
Flavonoid O-glycosides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Flavonoid o-glycoside
- Flavonoid-6-o-glycoside
- Methoxyflavonoid skeleton
- 7-methoxyflavonoid-skeleton
- Hydroxyflavonoid
- Flavone
- 5-hydroxyflavonoid
- Phenolic glycoside
- O-glycosyl compound
- Glycosyl compound
- Disaccharide
- Chromone
- 1-benzopyran
- Methoxyphenol
- Benzopyran
- Anisole
- Pyranone
- Alkyl aryl ether
- Benzenoid
- Pyran
- Oxane
- Saccharide
- Monocyclic benzene moiety
- Heteroaromatic compound
- Vinylogous acid
- Secondary alcohol
- Polyol
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Ether
- Acetal
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
238 - 239.3 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 238 - 239.3 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Nagashima S, Hirotani M, Yoshikawa T (2000)Purification and characterization of UDP-glucuronate: baicalein 7-O-glucuronosyltransferase from Scutellaria baicalensis Georgi. cell suspension cultures. Phytochemistry 53, Pubmed: 10724177
- Huang Y, Tsang SY, Yao X, Chen ZY (2005)Biological properties of baicalein in cardiovascular system. Current drug targets. Cardiovascular & haematological disorders 5, Pubmed: 15853750
- Sithisarn P, Michaelis M, Schubert-Zsilavecz M, Cinatl J (2013)Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral research 97, Pubmed: 23098745
- Oh SB, Park HR, Jang YJ, Choi SY, Son TG, Lee J (2013)Baicalein attenuates impaired hippocampal neurogenesis and the neurocognitive deficits induced by ?-ray radiation. British journal of pharmacology 168, Pubmed: 22891631
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|