|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120549 |
|---|
|
Identification |
|---|
| Name: |
dehydroepiandrosterone |
|---|
| Description: | An androstanoid that is androst-5-ene substituted by a β-hydroxy group at position 3 and an oxo group at position 17. It is a naturally occurring steroid hormone produced by the adrenal glands. |
|---|
|
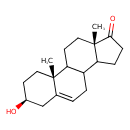
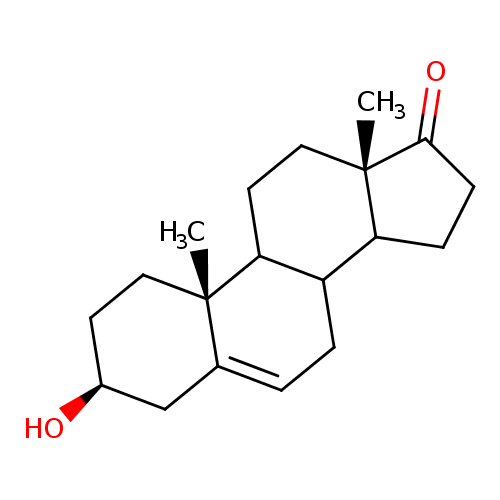
Structure |
|
|---|
| Synonyms: | - 3-BETA-HYDROXY-5-ANDROSTEN-17-ONE
- 3beta-hydroxyandrost-5-en-17-one
- 3beta-Hydroxyandrost-5-en-17-one
- 3β-hydroxyandrost-5-en-17-one
- 3beta-Hydroxyandrost-5-en-17-one
- Dehydroepiandrosterone
- Dehydroisoandrosterone
- DHA
- DHEA
- Prasterone
|
|---|
|
Chemical Formula: |
C19H28O2 |
|---|
| Average Molecular Weight: |
288.429 |
|---|
| Monoisotopic Molecular
Weight: |
288.20892 |
|---|
| InChI Key: |
FMGSKLZLMKYGDP-USOAJAOKSA-N |
|---|
| InChI: | InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h3,13-16,20H,4-11H2,1-2H3/t13-,14-,15-,16-,18-,19-/m0/s1 |
|---|
| CAS
number: |
53-43-0 |
|---|
| IUPAC Name: | 3β-hydroxyandrost-5-en-17-one |
|---|
|
Traditional IUPAC Name: |
dhea - dehydroepiandrosterone |
|---|
| SMILES: | CC24(CCC(O)CC(=CC[CH]1([CH]3(CCC(=O)C(CC[CH]12)(C)3)))4) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
|
Direct Parent |
Androgens and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Androgen-skeleton
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- Hydroxysteroid
- 17-oxosteroid
- Oxosteroid
- Delta-5-steroid
- Cyclic alcohol
- Ketone
- Secondary alcohol
- Organooxygen compound
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic homopolycyclic compounds |
|---|
| External Descriptors |
- a 3\u0026beta;-hydroxy-\u0026delta;\u003csup\u003e5\u003c/sup\u003e-steroid (3-BETA-HYDROXYANDROST-5-EN-17-ONE)
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
140 - 141 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 140 - 141 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.0635 mg/mL | Not Available | | LogP | 3.23 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Androgen and Estrogen Metabolism pae00150
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Ng MK, Nakhla S, Baoutina A, Jessup W, Handelsman DJ, Celermajer DS (2003)Dehydroepiandrosterone, an adrenal androgen, increases human foam cell formation: a potentially pro-atherogenic effect. Journal of the American College of Cardiology 42, Pubmed: 14662261
- Narkwichean A, Jayaprakasan K, Maalouf WE, Hernandez-Medrano JH, Pincott-Allen C, Campbell BK (2014)Effects of dehydroepiandrosterone on in vivo ovine follicular development. Human reproduction (Oxford, England) 29, Pubmed: 24256992
- Engdahl C, Lagerquist MK, Stubelius A, Andersson A, Studer E, Ohlsson C, Westberg L, Carlsten H, Forsblad-d'Elia H (2014)Role of androgen and estrogen receptors for the action of dehydroepiandrosterone (DHEA). Endocrinology 155, Pubmed: 24424045
- Krysiak R, Frysz-Naglak D, Okopien B (2008)[Current views on the role of dehydroepiandrosterone in physiology, pathology and therapy]. Polski merkuriusz lekarski : organ Polskiego Towarzystwa Lekarskiego 24, Pubmed: 18634257
|
|---|
| Synthesis Reference: |
Nguyen Xuan Cuong; Nguyen Van Dan. Synthesis of dehydroepiandrosterone (DHA) from 16-dehydropregnenolone acetate (DPA). Tap Chi Duoc Hoc (1983), (4), 12-14. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|