|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120547 |
|---|
|
Identification |
|---|
| Name: |
pristanoyl-CoA |
|---|
| Description: | A multi-methyl-branched fatty acyl-CoA(4-) oxanion arising from deprotonation of the phosphate and diphosphate OH groups of pristanoyl-CoA; major species at pH 7.3. |
|---|
|
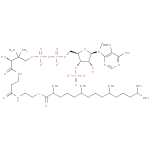
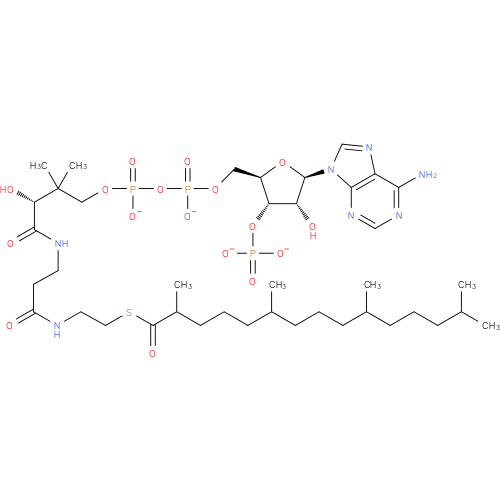
Structure |
|
|---|
| Synonyms: | - pristanoyl-CoA
- pristanoyl-coenzyme A(4−)
|
|---|
|
Chemical Formula: |
C40H68N7O17P3S |
|---|
| Average Molecular Weight: |
1043.995 |
|---|
| Monoisotopic Molecular
Weight: |
1047.3918 |
|---|
| InChI Key: |
XYJPSQPVCBNZHT-TUKYSRJDSA-J |
|---|
| InChI: | InChI=1S/C40H72N7O17P3S/c1-25(2)11-8-12-26(3)13-9-14-27(4)15-10-16-28(5)39(52)68-20-19-42-30(48)17-18-43-37(51)34(50)40(6,7)22-61-67(58,59)64-66(56,57)60-21-29-33(63-65(53,54)55)32(49)38(62-29)47-24-46-31-35(41)44-23-45-36(31)47/h23-29,32-34,38,49-50H,8-22H2,1-7H3,(H,42,48)(H,43,51)(H,56,57)(H,58,59)(H2,41,44,45)(H2,53,54,55)/p-4/t26?,27?,28?,29-,32-,33-,34+,38-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | Not Available |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC(C)CCCC(C)CCCC(C)CCCC(C)C(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(=O)(OP(=O)(OCC1(C(OP([O-])(=O)[O-])C(O)C(O1)N3(C2(=C(C(N)=NC=N2)N=C3))))[O-])[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as long-chain fatty acyl coas. These are acyl CoAs where the group acylated to the coenzyme A moiety is a long aliphatic chain of 13 to 21 carbon atoms. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
|
Direct Parent |
Long-chain fatty acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Diterpenoid
- Pentose phosphate
- Ribonucleoside 3'-phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- Organic pyrophosphate
- Monosaccharide phosphate
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Aminopyrimidine
- N-acyl-amine
- Imidolactam
- Alkyl phosphate
- N-substituted imidazole
- Organic phosphoric acid derivative
- Monosaccharide
- Pyrimidine
- Fatty amide
- Phosphoric acid ester
- Tetrahydrofuran
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Thiocarboxylic acid ester
- Carbothioic s-ester
- Carboxamide group
- Azacycle
- Organoheterocyclic compound
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Oxacycle
- Carboxylic acid derivative
- Organic oxide
- Organic oxygen compound
- Organic nitrogen compound
- Primary amine
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Amine
- Organopnictogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Westin MA, Hunt MC, Alexson SE (2007)Peroxisomes contain a specific phytanoyl-CoA/pristanoyl-CoA thioesterase acting as a novel auxiliary enzyme in alpha- and beta-oxidation of methyl-branched fatty acids in mouse. The Journal of biological chemistry 282, Pubmed: 17613526
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|