| References: |
- Narayanan J, Hayakawa Y, Fan J, Kirk KL (2003)Convenient syntheses of biogenic aldehydes, 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde. Bioorganic chemistry 31, Pubmed: 12729575
- Burke WJ, Schmitt CA, Gillespie KN, Li SW (1996)Norepinephrine transmitter metabolite is a selective cell death messenger in differentiated rat pheochromocytoma cells. Brain research 722, Pubmed: 8813375
- Kawamura M, Eisenhofer G, Kopin IJ, Kador PF, Lee YS, Tsai JY, Fujisawa S, Lizak MJ, Sinz A, Sato S (1999)Aldose reductase, a key enzyme in the oxidative deamination of norepinephrine in rats. Biochemical pharmacology 58, Pubmed: 10424772
- Dina OA, Khasar SG, Alessandri-Haber N, Bogen O, Chen X, Green PG, Reichling DB, Messing RO, Levine JD (2008)Neurotoxic catecholamine metabolite in nociceptors contributes to painful peripheral neuropathy. The European journal of neuroscience 28, Pubmed: 18783367
- Li SW, Lin TS, Minteer S, Burke WJ (2001)3,4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson's disease pathogenesis. Brain research. Molecular brain research 93, Pubmed: 11532332
- Burke WJ, Schmitt CA, Miller C, Li SW (1997)Norepinephrine transmitter metabolite induces apoptosis in differentiated rat pheochromocytoma cells. Brain research 760, Pubmed: 9237550
- Burke WJ, Kristal BS, Yu BP, Li SW, Lin TS (1998)Norepinephrine transmitter metabolite generates free radicals and activates mitochondrial permeability transition: a mechanism for DOPEGAL-induced apoptosis. Brain research 787, Pubmed: 9518674
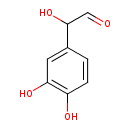
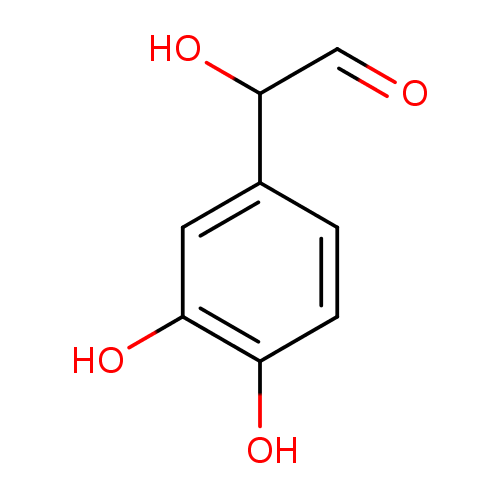
- Burke WJ, Li SW, Schmitt CA, Xia P, Chung HD, Gillespie KN (1999)Accumulation of 3,4-dihydroxyphenylglycolaldehyde, the neurotoxic monoamine oxidase A metabolite of norepinephrine, in locus ceruleus cell bodies in Alzheimer's disease: mechanism of neuron death. Brain research 816, Pubmed: 9878889
- Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, Zahm DS (2004)Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology 25, Pubmed: 14697885
|
|---|