|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120511 |
|---|
|
Identification |
|---|
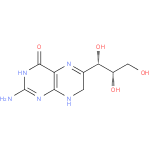
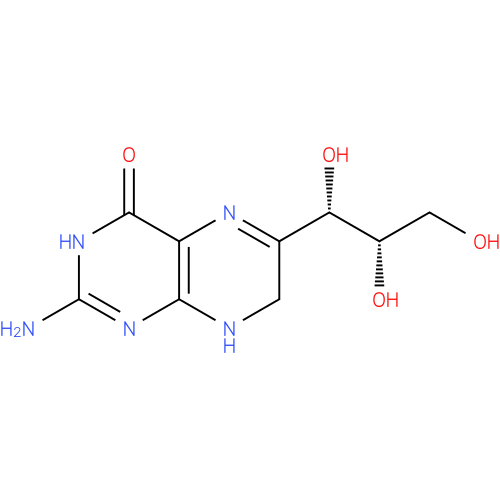
| Name: |
7,8-dihydromonapterin |
|---|
| Description: | A dihydropterin that is monapterin dihydrogenated at positions 7 and 8. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 7,8-dihydromonapterin
- DHM
- H2-MPt
|
|---|
|
Chemical Formula: |
C9H13N5O4 |
|---|
| Average Molecular Weight: |
255.233 |
|---|
| Monoisotopic Molecular
Weight: |
255.09676 |
|---|
| InChI Key: |
YQIFAMYNGGOTFB-NJGYIYPDSA-N |
|---|
| InChI: | InChI=1S/C9H13N5O4/c10-9-13-7-5(8(18)14-9)12-3(1-11-7)6(17)4(16)2-15/h4,6,15-17H,1-2H2,(H4,10,11,13,14,18)/t4-,6-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-amino-6-[(1S,2S)-1,2,3-trihydroxypropyl]-7,8-dihydropteridin-4(3H)-one |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C1(NC2(N=C(N)NC(=O)C(N=C1C(O)C(O)CO)=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
|
Direct Parent |
Biopterins and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Biopterin
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Pyrimidine
- Vinylogous amide
- Heteroaromatic compound
- Ketimine
- Secondary alcohol
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Polyol
- Amine
- Organopnictogen compound
- Primary amine
- Primary alcohol
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Imine
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- a small molecule (DIHYDRO-NEO-PTERIN)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Goyer A, Illarionova V, Roje S, Fischer M, Bacher A, Hanson AD (2004)Folate biosynthesis in higher plants. cDNA cloning, heterologous expression, and characterization of dihydroneopterin aldolases. Plant physiology 135, Pubmed: 15107504
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|