|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120509 |
|---|
|
Identification |
|---|
| Name: |
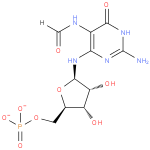
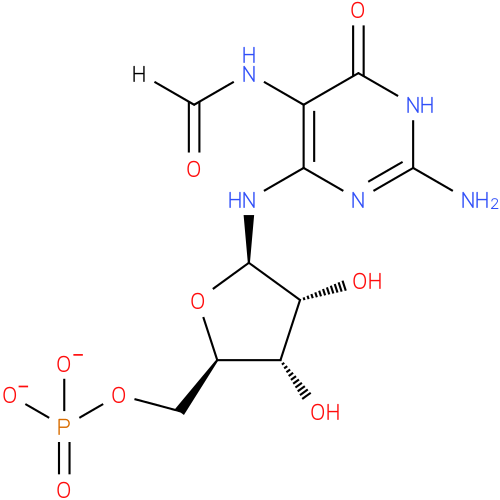
2-amino-5-formylamino-6-(5-phospho-D-ribosylamino)pyrimidin-4(3H)-one |
|---|
| Description: | The dianion obtained by removal of the two acidic protons from the phosphate group of 2-amino-5-formylamino-6-(1-D-ribosylamino)pyrimidin-4(3H)-one 5'-phosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-
 amino- amino- 5- 5- formylamino- formylamino- 6- 6- (1- (1- D- D- ribosylamino)- ribosylamino)- 4(3H)- 4(3H)- pyrimidinone 5'- pyrimidinone 5'- monophosphate(2−) monophosphate(2−) - 2-
 amino- amino- 5- 5- formylamino- formylamino- 6- 6- (1- (1- D- D- ribosylamino)- ribosylamino)- 4(3H)- 4(3H)- pyrimidinone 5'- pyrimidinone 5'- phosphate(2−) phosphate(2−) - 2-
 amino- amino- 5- 5- formylamino- formylamino- 6- 6- (1- (1- D- D- ribosylamino)pyrimidin- ribosylamino)pyrimidin- 4(3H)- 4(3H)- one 5'- one 5'- monophosphate(2−) monophosphate(2−) - 2-amino-5-formylamino-6-(5-phospho-D-ribosylamino)pyrimidin-4(3H)-one
- 2-amino-5-formylamino-6-ribosylamino-4(3H)-pyrimidinone 5'-monophosphate(2−)
- 2-amino-5-formylamino-6-ribosylamino-4(3H)-pyrimidinone 5'-phosphate(2−)
- FAPy
- N-
 (2- (2- amino- amino- 5- 5- formamido- formamido- 6- 6- oxo- oxo- 1,6- 1,6- dihydropyrimidin- dihydropyrimidin- 4- 4- yl)- yl)- 5- 5- O- O- phosphonato- phosphonato- β- β- D- D- ribofuranosylamine ribofuranosylamine
|
|---|
|
Chemical Formula: |
C10H14N5O9P |
|---|
| Average Molecular Weight: |
379.222 |
|---|
| Monoisotopic Molecular
Weight: |
381.06857 |
|---|
| InChI Key: |
VKMYTDDVUBGBDH-UUOKFMHZSA-L |
|---|
| InChI: | InChI=1S/C10H16N5O9P/c11-10-14-7(4(12-2-16)8(19)15-10)13-9-6(18)5(17)3(24-9)1-23-25(20,21)22/h2-3,5-6,9,17-18H,1H2,(H,12,16)(H2,20,21,22)(H4,11,13,14,15,19)/p-2/t3-,5-,6-,9-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 1- deoxy- deoxy- 1- 1- [(2- [(2- amino- amino- 5- 5- formamido- formamido- 6- 6- oxo- oxo- 1,6- 1,6- dihydropyrimidin- dihydropyrimidin- 4- 4- yl)amino]- yl)amino]- β- β- D- D- ribofuranose 5- ribofuranose 5- phosphate phosphate |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C1(OC(C(O)C(O)1)NC2(N=C(N)NC(=O)C(NC=O)=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
|
Class |
Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
|
Direct Parent |
Pentose phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- N-arylamide
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Hydropyrimidine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Imidolactam
- Vinylogous amide
- Tetrahydrofuran
- Heteroaromatic compound
- Secondary alcohol
- Amino acid or derivatives
- Secondary carboxylic acid amide
- Carboxamide group
- Lactam
- 1,2-diol
- Secondary amine
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Organic nitrogen compound
- Primary amine
- Organic oxide
- Organopnictogen compound
- Hydrocarbon derivative
- Alcohol
- Carbonyl group
- Organonitrogen compound
- Amine
- Organic anion
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Grochowski LL, Xu H, White RH (2009)An iron(II) dependent formamide hydrolase catalyzes the second step in the archaeal biosynthetic pathway to riboflavin and 7,8-didemethyl-8-hydroxy-5-deazariboflavin. Biochemistry 48, Pubmed: 19309161
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|