|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120484 |
|---|
|
Identification |
|---|
| Name: |
taxiphyllin |
|---|
| Description: | A β-D-glucoside consisting of (R)-prunasin carrying a hydroxy substituent at position 4 on the phenyl ring. |
|---|
|
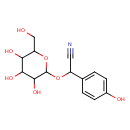
Structure |
|
|---|
| Synonyms: | - (R)-4-Hydroxymandelonitrile beta-D-glucoside
- (R)-alpha-(β-D-glucopyranosyloxy)-4-hydroxybenzeneacetonitrile
- (R)-p-hydroxymandelonitrile-D-glucopyranoside
- Taxiphyllin
|
|---|
|
Chemical Formula: |
C14H17NO7 |
|---|
| Average Molecular Weight: |
311.291 |
|---|
| Monoisotopic Molecular
Weight: |
311.1005 |
|---|
| InChI Key: |
NVLTYOJHPBMILU-GMDXDWKASA-N |
|---|
| InChI: | InChI=1S/C14H17NO7/c15-5-9(7-1-3-8(17)4-2-7)21-14-13(20)12(19)11(18)10(6-16)22-14/h1-4,9-14,16-20H,6H2/t9-,10+,11+,12-,13+,14+/m0/s1 |
|---|
| CAS
number: |
21401-21-8 |
|---|
| IUPAC Name: | (2R)-(β-D-glucopyranosyloxy)(4-hydroxyphenyl)acetonitrile |
|---|
|
Traditional IUPAC Name: |
dhurrin |
|---|
| SMILES: | C(C(C1(C=CC(=CC=1)O))OC2(OC(C(C(C2O)O)O)CO))#N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as cyanogenic glycosides. These are glycosides in which the aglycone moiety contains a cyanide group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Cyanogenic glycosides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Cyanogenic glycoside
- Hexose monosaccharide
- O-glycosyl compound
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Monosaccharide
- Oxane
- Benzenoid
- Secondary alcohol
- Polyol
- Oxacycle
- Nitrile
- Carbonitrile
- Acetal
- Organoheterocyclic compound
- Organonitrogen compound
- Hydrocarbon derivative
- Alcohol
- Organopnictogen compound
- Organic nitrogen compound
- Primary alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
176 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 176 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0w29-0922000000-4323039c23c09ae7d5b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0900000000-20291a7cc72858475773 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ff0-2900000000-d9b8829840517b5ef00a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dj-1927000000-2051e5cb0bd7423a8dad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-2910000000-abc72215556e260df8c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-8900000000-11bb86435bb0e9209de4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0w29-0922000000-4323039c23c09ae7d5b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0900000000-20291a7cc72858475773 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ff0-2900000000-d9b8829840517b5ef00a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dj-1927000000-2051e5cb0bd7423a8dad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-2910000000-abc72215556e260df8c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-8900000000-11bb86435bb0e9209de4 | View in MoNA |
|---|
|
|---|
|
References |
|---|
| References: |
- Petruccioli M, Brimer L, Cicalini AR, Pulci V, Federici F (1999)Production and properties of the linamarase and amygdalase activities of Penicillium aurantiogriseum P35. Bioscience, biotechnology, and biochemistry 63, Pubmed: 10380623
- Calderón AI, Terreaux C, Gupta MP, Hostettmann K, Schenk KJ (2003)Taxiphyllin from Henriettella fascicularis. Acta crystallographica. Section C, Crystal structure communications 59, Pubmed: 12711800
- Rosen MA, Farnden KJ, Conn EE (1975)Stereochemical aspects of the biosynthesis of the epimeric cyanogenic glucosides dhurrin and taxiphyllin. The Journal of biological chemistry 250, Pubmed: 1194256
- Nahrstedt A, Kant JD, Hösel W (1984)Aspects on the Biosynthesis of the Cyanogenic Glucoside Triglochinin in Triglochin maritima1. Planta medica 50, Pubmed: 17340339
- Cutler AJ, Hösel W, Sternberg M, Conn EE (1981)The in vitro biosynthesis of taxiphyllin and the channeling of intermediates in Triglochin maritima. The Journal of biological chemistry 256, Pubmed: 7012151
- Oueslati MH, Ben Jannet H, Mighri Z, Chriaa J, Abreu PM (2006)Phytochemical constituents from Salsola tetrandra. Journal of natural products 69, Pubmed: 16989538
- Hösel W, Nahrstedt A (1980)In vitro biosynthesis of the cyanogenic glucoside taxiphyllin in Triglochin maritima. Archives of biochemistry and biophysics 203, Pubmed: 7458352
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|