|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120477 |
|---|
|
Identification |
|---|
| Name: |
D-arabitol |
|---|
| Description: | The D-enantiomer of arabinitol. |
|---|
|
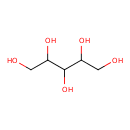
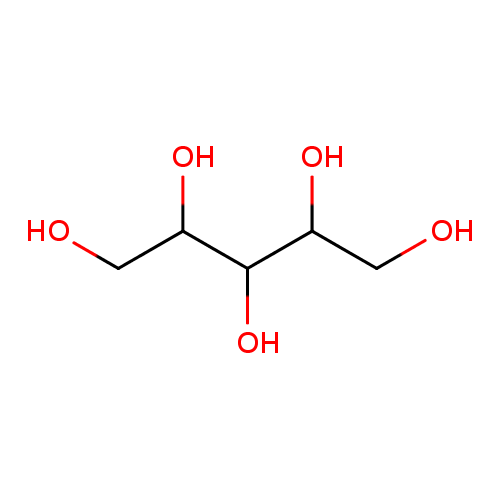
Structure |
|
|---|
| Synonyms: | - D-Arabinitol
- D-arabinitol
- D-Arabinol
- D-Arabitol
- D-Lyxitol
|
|---|
|
Chemical Formula: |
C5H12O5 |
|---|
| Average Molecular Weight: |
152.147 |
|---|
| Monoisotopic Molecular
Weight: |
152.06847 |
|---|
| InChI Key: |
HEBKCHPVOIAQTA-QWWZWVQMSA-N |
|---|
| InChI: | InChI=1S/C5H12O5/c6-1-3(8)5(10)4(9)2-7/h3-10H,1-2H2/t3-,4-/m1/s1 |
|---|
| CAS
number: |
488-82-4 |
|---|
| IUPAC Name: | D-arabinitol |
|---|
|
Traditional IUPAC Name: |
1,2,3,4,5-pentahydroxypentane |
|---|
| SMILES: | C(O)C(O)C(O)C(O)CO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as sugar alcohols. These are hydrogenated forms of carbohydrate in which the carbonyl group (aldehyde or ketone, reducing sugar) has been reduced to a primary or secondary hydroxyl group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Sugar alcohols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Sugar alcohol
- Monosaccharide
- Secondary alcohol
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
101 - 104 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 101 - 104 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 729 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-01ba-9500000000-67233150807800e941f0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-01b9-9000000000-1ff17a8b87bbab9a3622 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-044i-9000000000-e0e330c8a899be718f60 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1900000000-88c39e799daf7fb9dbca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9300000000-66487a26d7047f9096a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-9000000000-514c2ae8b98eb4994147 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udu-9400000000-b3ca0d23907878fbe1a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0btl-9200000000-92a36674373a271da867 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0btc-9000000000-5e7378c18473f8226282 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-01vo-9000000000-f065454e8412613f8ecd | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Sánchez-Fresneda R, Guirao-Abad JP, Argüelles A, González-Párraga P, Valentín E, Argüelles JC (2013)Specific stress-induced storage of trehalose, glycerol and D-arabitol in response to oxidative and osmotic stress in Candida albicans. Biochemical and biophysical research communications 430, Pubmed: 23261427
- Roboz J (1994)Diagnosis and monitoring of disseminated candidiasis based on serum/urine D/L-arabinitol ratios. Chirality 6, Pubmed: 8204415
- Sigmundsdóttir G, Christensson B, Björklund LJ, Håkansson K, Pehrson C, Larsson L (2000)Urine D-arabinitol/L-arabinitol ratio in diagnosis of invasive candidiasis in newborn infants. Journal of clinical microbiology 38, Pubmed: 10921974
|
|---|
| Synthesis Reference: |
Delobeau, Didier; Moine, Didier. Process for the preparation of d-arabitol from lactose. Eur. Pat. Appl. (1997), 9 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|