|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120452 |
|---|
|
Identification |
|---|
| Name: |
n-butanol |
|---|
| Description: | A primary alcohol that is butane in which a hydrogen of one of the methyl groups is substituted by a hydroxy group. It it produced in small amounts in humans by the gut microbes. |
|---|
|
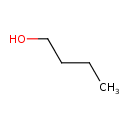
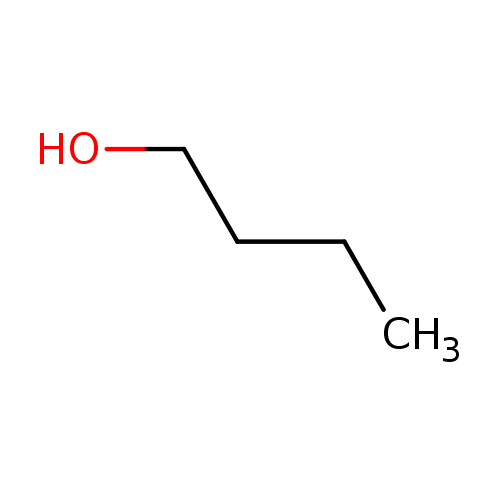
Structure |
|
|---|
| Synonyms: | - 1-Butanol
- 1-BUTANOL
- 1-butyl alcohol
- 1-hydroxybutane
- BuOH
- butan-1-ol
- n-butan-1-ol
- n-Butanol
- n-butyl alcohol
- n-Butylalkohol
- propyl carbinol
|
|---|
|
Chemical Formula: |
C4H10O |
|---|
| Average Molecular Weight: |
74.122 |
|---|
| Monoisotopic Molecular
Weight: |
74.073166 |
|---|
| InChI Key: |
LRHPLDYGYMQRHN-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H10O/c1-2-3-4-5/h5H,2-4H2,1H3 |
|---|
| CAS
number: |
71-36-3 |
|---|
| IUPAC Name: | butan-1-ol |
|---|
|
Traditional IUPAC Name: |
butanol |
|---|
| SMILES: | CCCCO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as primary alcohols. These are compounds comprising the primary alcohol functional group, with the general structure RCOH (R=alkyl, aryl). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
|
Class |
Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
|
Direct Parent |
Primary alcohols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydrocarbon derivative
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
-89.8 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | -89.8 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 63.2 mg/mL at 25 °C | Not Available | | LogP | 0.88 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-6M) , Positive | splash10-052f-9000000000-69148177e3417cc669fd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7M) , Positive | splash10-052f-9000000000-7ebca2648ffd63942337 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80B) , Positive | splash10-055f-9000000000-7e23cd40dde01cb89b6f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-9000000000-53a465f38bad658bb673 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-9145b21c5f3ae37774e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-686e7cdc50d3fda3a0ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-9000000000-53a465f38bad658bb673 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-9145b21c5f3ae37774e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-686e7cdc50d3fda3a0ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9000000000-61d09b30d6a547d109fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-4d4d1c2159c658c4ee24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-2119b542a1efb8432d12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9000000000-61d09b30d6a547d109fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-4d4d1c2159c658c4ee24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-2119b542a1efb8432d12 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-054o-9000000000-061591a78bc1cdeda799 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Frederickx C, Dekeirsschieter J, Verheggen FJ, Haubruge E (2014)Host-habitat location by the parasitoid, Nasonia vitripennis Walker (Hymenoptera: Pteromalidae). Journal of forensic sciences 59, Pubmed: 23980702
- Liebich HM, Buelow HJ, Kallmayer R (1982)Quantification of endogenous aliphatic alcohols in serum and urine. Journal of chromatography 239, Pubmed: 7096503
|
|---|
| Synthesis Reference: |
Tsuchida, Takashi; Sakuma, Shuji. Ethanol to 1-butanol on hydroxyapatite. Shokubai (2007), 49(3), 238-243. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|