|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120438 |
|---|
|
Identification |
|---|
| Name: |
3-methylbutanal |
|---|
| Description: | A methylbutanal that is butanal substituted by a methyl group at position 3. It occurs as a volatile constituent in olives. |
|---|
|
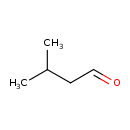
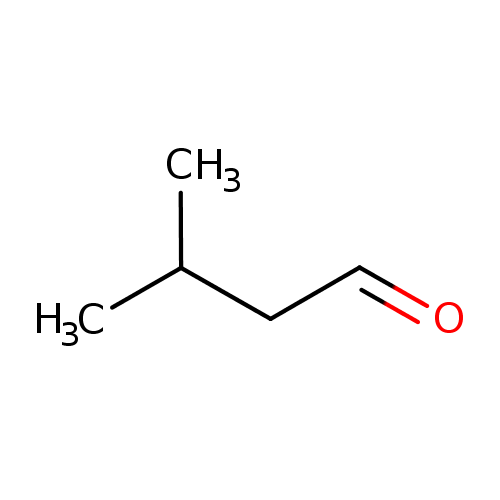
Structure |
|
|---|
| Synonyms: | - 3-Methylbutanal
- 3-methylbutanal
- 3-methylbutyraldehyde
- β-Methylbutanal
- iso-C4H9CHO
- Isoamyl aldehyde
- Isopentaldehyde
- Isovaleral
- Isovaleraldehyde
- Isovalerylaldehyde
|
|---|
|
Chemical Formula: |
C5H10O |
|---|
| Average Molecular Weight: |
86.133 |
|---|
| Monoisotopic Molecular
Weight: |
86.073166 |
|---|
| InChI Key: |
YGHRJJRRZDOVPD-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C5H10O/c1-5(2)3-4-6/h4-5H,3H2,1-2H3 |
|---|
| CAS
number: |
590-86-3 |
|---|
| IUPAC Name: | 3-methylbutanal |
|---|
|
Traditional IUPAC Name: |
isovaleraldehyde |
|---|
| SMILES: | CC(C)CC=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as alpha-hydrogen aldehydes. These are aldehydes with the general formula HC(H)(R)C(=O)H, where R is an organyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
|
Class |
Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
|
Direct Parent |
Alpha-hydrogen aldehydes |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-hydrogen aldehyde
- Organic oxide
- Hydrocarbon derivative
- Short-chain aldehyde
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
- methylbutanal (CHEBI:16638)
- a small molecule (CPD-7031)
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
-51 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | -51 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 14 mg/mL at 20 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-9000000000-7f4ac528265c8b3bf3ab | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-9000000000-6e4f25dc68765a0e52ca | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-000i-9000000000-809684cd2152204e911f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-6M) , Positive | splash10-0006-9000000000-3a38c8f6150bf1f43e74 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-6M) , Positive | splash10-0006-9000000000-5a3fd4f38dc36ebe58a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9000000000-a8ed71d6e7d567b598b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kp-9000000000-043451832d2f37b1c7e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-3c57d920ca714b174bb3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-030536579800c9e49940 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9000000000-be1f1229d7e13994d150 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-13768c049fd8e5c23464 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9000000000-a8ed71d6e7d567b598b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kp-9000000000-043451832d2f37b1c7e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-3c57d920ca714b174bb3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-030536579800c9e49940 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9000000000-be1f1229d7e13994d150 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-13768c049fd8e5c23464 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0006-9000000000-881fcc89b0f579b40793 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Sansone-Land A, Takeoka GR, Shoemaker CF (2014)Volatile constituents of commercial imported and domestic black-ripe table olives (Olea europaea). Food chemistry 149, Pubmed: 24295708
- Afzal MI, Boulahya KA, Paris C, Delaunay S, Cailliez-Grimal C (2013)Effect of oxygen on the biosynthesis of flavor compound 3-methylbutanal from leucine catabolism during batch culture in Carnobacterium maltaromaticum LMA 28. Journal of dairy science 96, Pubmed: 23182362
|
|---|
| Synthesis Reference: |
Roelen, O. Synthesis of aldehydes and derivatives from olefins, carbon monoxide, and hydrogen. Angew. Chem. (1948), A60 62. CAN 44:46679 AN 1950:46679 |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|