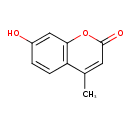
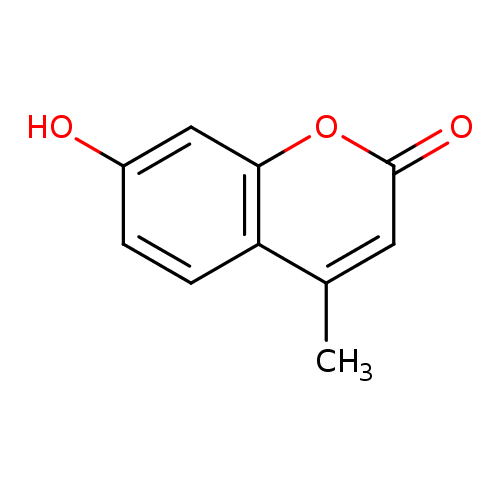
| References: |
- Nakamura T, Ishikawa T, Nanashima N, Miura T, Nozaka H, Nakaoka R, Sato T (2002)4-Methylumbelliferone induces the expression of membrane type 1-matrix metalloproteinase in cultured human skin fibroblasts. Biochemical and biophysical research communications 298, Pubmed: 12419303
- Varga JM, Kalchschmid G, Klein GF, Fritsch P (1991)Mechanism of allergic cross-reactions--I. Multispecific binding of ligands to a mouse monoclonal anti-DNP IgE antibody. Molecular immunology 28, Pubmed: 1650428
- Lokeshwar VB, Lopez LE, Munoz D, Chi A, Shirodkar SP, Lokeshwar SD, Escudero DO, Dhir N, Altman N (2010)Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer research 70, Pubmed: 20332231
- Nakamura R, Kuwabara H, Yoneda M, Yoshihara S, Ishikawa T, Miura T, Nozaka H, Nanashima N, Sato T, Nakamura T (2007)Suppression of matrix metalloproteinase-9 by 4-methylumbelliferone. Cell biology international 31, Pubmed: 17470403
- Saito T, Tamura D, Nakamura T, Makita Y, Ariyama H, Komiyama K, Yoshihara T, Asano R (2013)4-methylumbelliferone leads to growth arrest and apoptosis in canine mammary tumor cells. Oncology reports 29, Pubmed: 23124556
- Fang Y, Wang H, Zhu W, Wang L, Liu H, He Y, Xu X, Yin W, Sima Y, Xu S (2014)Antioxidative capacity in the fat body of Bombyx mori is increased following oral administration of 4-methylumbelliferone. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP 159, Pubmed: 24080584
- Qin J, Kilkus J, Dawson G (2016)The hyaluronic acid inhibitor 4-methylumbelliferone is an NSMase2 activator-role of Ceramide in MU anti-tumor activity. Biochimica et biophysica acta 1861, Pubmed: 26548718
- Benitez A, Yates TJ, Shamaldevi N, Bowen T, Lokeshwar VB (2013)Dietary supplement hymecromone and sorafenib: a novel combination for the control of renal cell carcinoma. The Journal of urology 190, Pubmed: 23228386
|
|---|