|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120415 |
|---|
|
Identification |
|---|
| Name: |
malonate |
|---|
| Description: | A dicarboxylic acid dianion obtained by the deprotonation of the carboxy groups of malonic acid. |
|---|
|
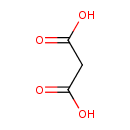
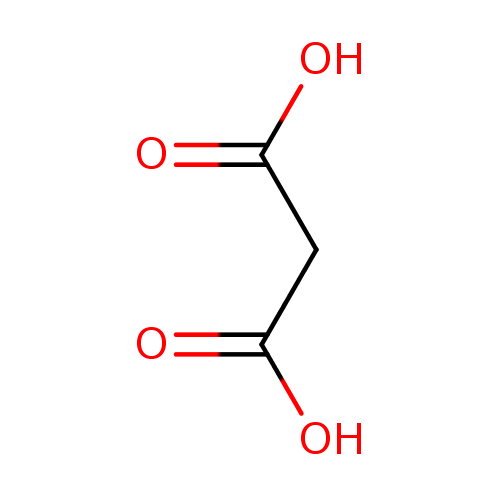
Structure |
|
|---|
| Synonyms: | - malo
- malonate
- MALONATE ION
- malonic acid, ion(2−)
- −OOC‒CH2‒COO−
- propanedioic acid, ion(2−)
|
|---|
|
Chemical Formula: |
C3H2O4 |
|---|
| Average Molecular Weight: |
102.046 |
|---|
| Monoisotopic Molecular
Weight: |
104.010956 |
|---|
| InChI Key: |
OFOBLEOULBTSOW-UHFFFAOYSA-L |
|---|
| InChI: | InChI=1S/C3H4O4/c4-2(5)1-3(6)7/h1H2,(H,4,5)(H,6,7)/p-2 |
|---|
| CAS
number: |
141-82-2 |
|---|
| IUPAC Name: | propanedioate |
|---|
|
Traditional IUPAC Name: |
malonic acid |
|---|
| SMILES: | C(C([O-])=O)C([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as dicarboxylic acids and derivatives. These are organic compounds containing exactly two carboxylic acid groups. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Dicarboxylic acids and derivatives |
|---|
|
Direct Parent |
Dicarboxylic acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 1,3-dicarbonyl compound
- Dicarboxylic acid or derivatives
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
135 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 135 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 763.0 mg/mL | Not Available | | LogP | -0.81 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0002-1900000000-a1463432c138c328557d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0002-0900000000-5e58241137fee0ccc64a | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-006t-9700000000-54976b3ce8f36ce0676d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-001i-4930000000-e7dbed4919870db8dabf | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-05gl-1943000000-456e387fdf365bf0f8ee | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-0a4i-9100000000-c7a1704f8a38ca2d245a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-0a4l-9100000000-9fa84f43f2e19b56035c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-052f-9100000000-8a21753b343cf5dabdcb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (Unknown) , Positive | splash10-0006-9000000000-6c56a402111059603ba4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7M) , Positive | splash10-0006-9000000000-f239df8fdd12e9a74445 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0udi-3900000000-e0a4c7e792cfd0e60cc3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0a4i-9000000000-4c03aa889e6a98ab532f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0a4l-9000000000-b3d14c986f292bdcf477 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0006-9000000000-8e1041322f9acda4088a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0006-9000000000-af4dccf21d68110099a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-01ox-9000000000-dd4efef191376724d0f1 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Ohdoi C, Nyhan WL, Kuhara T: Chemical diagnosis of Lesch-Nyhan syndrome using gas chromatography-mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 15;792(1):123-30. [12829005 ]
- Yano S, Sweetman L, Thorburn DR, Mofidi S, Williams JC: A new case of malonyl coenzyme A decarboxylase deficiency presenting with cardiomyopathy. Eur J Pediatr. 1997 May;156(5):382-3. [9177981 ]
- Belgorodsky B, Fadeev L, Ittah V, Benyamini H, Zelner S, Huppert D, Kotlyar AB, Gozin M: Formation and characterization of stable human serum albumin-tris-malonic acid [C60]fullerene complex. Bioconjug Chem. 2005 Sep-Oct;16(5):1058-62. [16173780 ]
- Buyukgebiz B, Jakobs C, Scholte HR, Huijmans JG, Kleijer WJ: Fatal neonatal malonic aciduria. J Inherit Metab Dis. 1998 Feb;21(1):76-7. [9501274 ]
- Haan EA, Scholem RD, Croll HB, Brown GK: Malonyl coenzyme A decarboxylase deficiency. Clinical and biochemical findings in a second child with a more severe enzyme defect. Eur J Pediatr. 1986 Apr;144(6):567-70. [3709568 ]
- Pollitt RJ, Fowler B, Sardharwalla IB, Edwards MA, Gray RG: Increased excretion of propan-1,3-diol and 3-hydroxypropionic acid apparently caused by abnormal bacterial metabolism in the gut. Clin Chim Acta. 1987 Nov 16;169(2-3):151-7. [3427776 ]
- Honda A, Yamashita K, Ikegami T, Hara T, Miyazaki T, Hirayama T, Numazawa M, Matsuzaki Y: Highly-sensitive quantification of serum malonate, a possible marker for de novo lipogenesis, by LC-ESI-MS/MS. J Lipid Res. 2009 Apr 29. [19403942 ]
|
|---|
| Synthesis Reference: |
Behr, Arno; Botulinski, Andreas; Carduck, Franz Josef; Schneider, Michael. Process for preparation of malonic acid. Ger. Offen. (1992), 4 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|