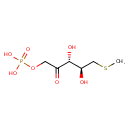|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120394 |
|---|
|
Identification |
|---|
| Name: |
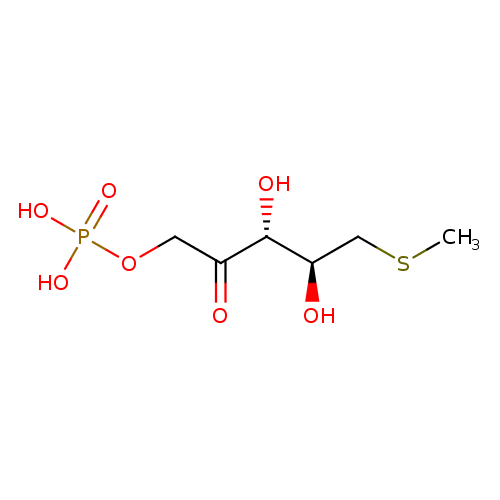
5-methylthioribulose 1-phosphate |
|---|
| Description: | Dianion of S-methyl-5-thio-D-ribulose 1-phosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 5-methylthio-5-deoxy-D-ribulose 1-phosphate
- 1-phosphomethylthioribulose
- methylthioribulose 1-phosphate
- MTRu-1-P1
- PMT-ribulose
- 1-phospho-5-S-methylthioribulose
|
|---|
|
Chemical Formula: |
C6H11O7PS |
|---|
| Average Molecular Weight: |
258.182 |
|---|
| Monoisotopic Molecular
Weight: |
260.01196 |
|---|
| InChI Key: |
CNSJRYUMVMWNMC-RITPCOANSA-L |
|---|
| InChI: | InChI=1S/C6H13O7PS/c1-15-3-5(8)6(9)4(7)2-13-14(10,11)12/h5-6,8-9H,2-3H2,1H3,(H2,10,11,12)/p-2/t5-,6+/m1/s1 |
|---|
| CAS
number: |
86316-83-8 |
|---|
| IUPAC Name: | 5-S-methyl-1-O-phosphonato-5-thio-D-ribulose |
|---|
|
Traditional IUPAC Name: |
methylthioribulose-1-phosphate |
|---|
| SMILES: | CSCC(O)C(O)C(=O)COP([O-])(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as glycerone phosphates. These are organic compounds containing a glycerone moiety that carries a phosphate group at the O-1 or O-2 position. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Glycerone phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Glycerone phosphate
- Monoalkyl phosphate
- Acyloin
- Beta-hydroxy ketone
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Alpha-hydroxy ketone
- Secondary alcohol
- 1,2-diol
- Thioether
- Sulfenyl compound
- Dialkylthioether
- Organosulfur compound
- Alcohol
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- S-methyl-5-thio-α-D-ribose 1-phosphate degradation IPWY-6755
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Ghoda LY, Savarese TM, Dexter DL, Parks RE Jr, Trackman PC, Abeles RH: Characterization of a defect in the pathway for converting 5'-deoxy-5'-methylthioadenosine to methionine in a subline of a cultured heterogeneous human colon carcinoma. J Biol Chem. 1984 Jun 10;259(11):6715-9. [6725268 ]
|
|---|
| Synthesis Reference: |
Imker, Heidi J.; Fedorov, Alexander A.; Fedorov, Elena V.; Almo, Steven C.; Gerlt, John A. Mechanistic Diversity in the RuBisCO Superfamily: The "Enolase" in the Methionine Salvage Pathway in Geobacillus kaustophilus. Biochemistry (2007), 46(13), 4077-4089. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|