|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120383 |
|---|
|
Identification |
|---|
| Name: |
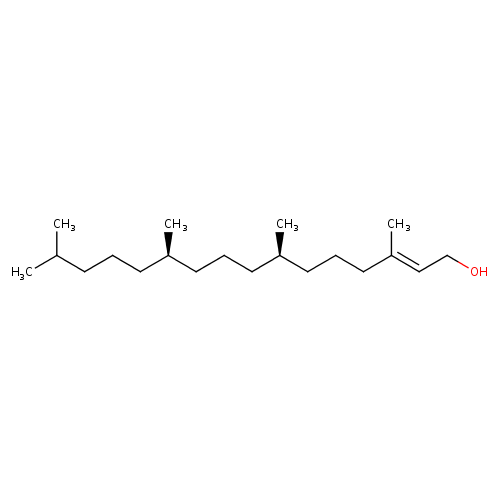
phytol |
|---|
| Description: | A diterpenoid that is hexadec-2-en-1-ol substituted by methyl groups at positions 3, 7, 11 and 15. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-ol
- Phytol
- phytol
- trans-Phytol
|
|---|
|
Chemical Formula: |
C20H40O |
|---|
| Average Molecular Weight: |
296.535 |
|---|
| Monoisotopic Molecular
Weight: |
296.30792 |
|---|
| InChI Key: |
BOTWFXYSPFMFNR-PYDDKJGSSA-N |
|---|
| InChI: | InChI=1S/C20H40O/c1-17(2)9-6-10-18(3)11-7-12-19(4)13-8-14-20(5)15-16-21/h15,17-19,21H,6-14,16H2,1-5H3/b20-15+/t18-,19-/m1/s1 |
|---|
| CAS
number: |
150-86-7 |
|---|
| IUPAC Name: | (2E,7R,11R)-3,7,11,15-tetramethylhexadec-2-en-1-ol |
|---|
|
Traditional IUPAC Name: |
phytol |
|---|
| SMILES: | CC(C)CCCC(C)CCCC(C)CCCC(C)=CCO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as acyclic diterpenoids. These are diterpenoids (compounds made of four consecutive isoprene units) that do not contain a cycle. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Prenol lipids |
|---|
|
Direct Parent |
Acyclic diterpenoids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Acyclic diterpenoid
- Long chain fatty alcohol
- Fatty alcohol
- Fatty acyl
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
< 25 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | < 25 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Costa JP, de Oliveira GA, de Almeida AA, Islam MT, de Sousa DP, de Freitas RM (2014)Anxiolytic-like effects of phytol: possible involvement of GABAergic transmission. Brain research 1547, Pubmed: 24333358
- Gloerich J, van den Brink DM, Ruiter JP, van Vlies N, Vaz FM, Wanders RJ, Ferdinandusse S (2007)Metabolism of phytol to phytanic acid in the mouse, and the role of PPARalpha in its regulation. Journal of lipid research 48, Pubmed: 17015885
- de Moraes J, de Oliveira RN, Costa JP, Junior AL, de Sousa DP, Freitas RM, Allegretti SM, Pinto PL (2014)Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLoS neglected tropical diseases 8, Pubmed: 24392173
- Pejin B, Savic A, Sokovic M, Glamoclija J, Ciric A, Nikolic M, Radotic K, Mojovic M (2014)Further in vitro evaluation of antiradical and antimicrobial activities of phytol. Natural product research 28, Pubmed: 24422895
|
|---|
| Synthesis Reference: |
Gramatica, Paola; Manitto, Paolo; Monti, Diego; Speranza, Giovanna. Microbial-mediated syntheses of EPC. Part 4. Stereoselective total synthesis of natural phytol via double bond reductions by baker's yeast. Tetrahedron (1987), 43(19), 4481-6. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|