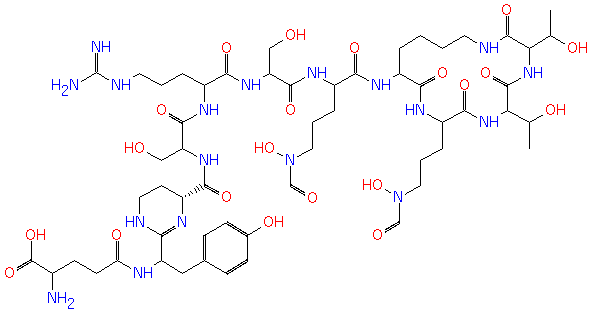
ferribactin (PAMDB120379)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB120379 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | ferribactin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Ferribactin is an intermediate in the biosynthesis of pyoverdine I, the type of pyoverdine that is synthesized by Pseudomonas aeruginosa . | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C56H90N18O21 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 1351.435 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 1350.6528 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | XXKMKYGWXRCAHF-DTIVLPDMSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C56H90N18O21/c1-29(79)43-53(90)61-19-4-3-8-34(46(83)66-37(11-7-23-74(95)28-78)50(87)71-44(30(2)80)54(91)72-43)65-47(84)36(10-6-22-73(94)27-77)68-52(89)40(25-75)69-48(85)35(9-5-20-62-56(58)59)67-51(88)41(26-76)70-49(86)38-18-21-60-45(64-38)39(24-31-12-14-32(81)15-13-31)63-42(82)17-16-33(57)55(92)93/h12-15,27-30,33-41,43-44,75-76,79-81,94-95H,3-11,16-26,57H2,1-2H3,(H,60,64)(H,61,90)(H,63,82)(H,65,84)(H,66,83)(H,67,88)(H,68,89)(H,69,85)(H,70,86)(H,71,87)(H,72,91)(H,92,93)(H4,58,59,62)/t29?,30?,33?,34?,35?,36?,37?,38-,39?,40?,41?,43?,44?/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(O)C1(NC(C(NC(=O)C(CCCN(C=O)O)NC(=O)C(CCCCNC(=O)1)NC(=O)C(CCCN(C=O)O)NC(=O)C(CO)NC(=O)C(NC(C(CO)NC(C3(CCNC(C(NC(CCC(C(=O)O)N)=O)CC2(C=CC(O)=CC=2))=N3))=O)=O)CCCNC(=N)N)C(C)O)=O) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as cyclic peptides. These are compounds containing a cyclic moiety bearing a peptide backbone. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Amino acids, peptides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Cyclic peptides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||