|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120375 |
|---|
|
Identification |
|---|
| Name: |
reduced riboflavin |
|---|
| Description: | Riboflavin in which the double bond between positions 4a and 5 has been reduced to a single bond. |
|---|
|
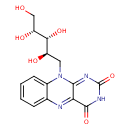
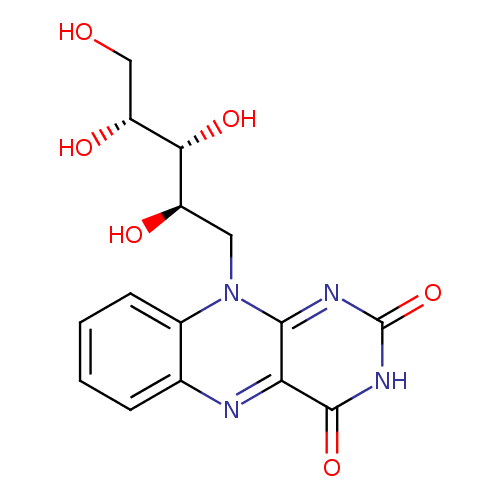
Structure |
|
|---|
| Synonyms: | - 4a,5-dihydroriboflavine
- 7,8-dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)-4a,5-dihydroisoalloxazine
- 7,8-
 dimethyl- dimethyl- 10- 10- (D- (D- ribo- ribo- 2,3,4,5- 2,3,4,5- tetrahydroxypentyl)- tetrahydroxypentyl)- 5,10- 5,10- dihydrobenzo[g]pteridine- dihydrobenzo[g]pteridine- 2,4(3H,4aH)- 2,4(3H,4aH)- dione dione
|
|---|
|
Chemical Formula: |
C17H22N4O6 |
|---|
| Average Molecular Weight: |
378.384 |
|---|
| Monoisotopic Molecular
Weight: |
378.15393 |
|---|
| InChI Key: |
UTKDOUCGQVLJIN-QNMSZWNNSA-N |
|---|
| InChI: | InChI=1S/C17H22N4O6/c1-7-3-9-10(4-8(7)2)21(5-11(23)14(25)12(24)6-22)15-13(18-9)16(26)20-17(27)19-15/h3-4,11-14,18,22-25H,5-6H2,1-2H3,(H,20,26,27)/t11-,12+,13?,14-/m1/s1 |
|---|
| CAS
number: |
101652-10-2 |
|---|
| IUPAC Name: | 1- deoxy- deoxy- 1- 1- {7,8- {7,8- dimethyl- dimethyl- 2,4- 2,4- dioxo- dioxo- 3,4,4a,5- 3,4,4a,5- tetrahydrobenzo[g]pteridin- tetrahydrobenzo[g]pteridin- 10(2H)- 10(2H)- yl}- yl}- D- D- ribitol ribitol |
|---|
|
Traditional IUPAC Name: |
10-[(2R,3R,4R)-2,3,4,5-tetrahydroxypentyl]-3H-benzo[g]pteridine-2,4-dione |
|---|
| SMILES: | CC1(=C(C=C2(C(=C1)NC3(C(N2CC(C(C(CO)O)O)O)=NC(NC3=O)=O)))C) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as alloxazines and isoalloxazines. These are organic compounds comprising the (iso)alloxazine structure (Benzo[g]pteridine-2,4-dione). |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Pteridines and derivatives |
|---|
|
Direct Parent |
Alloxazines and isoalloxazines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Isoalloxazine
- Diazanaphthalene
- Quinoxaline
- Pyrimidone
- Pyrazine
- Pyrimidine
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Secondary alcohol
- Lactam
- Polyol
- Azacycle
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|