|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120292 |
|---|
|
Identification |
|---|
| Name: |
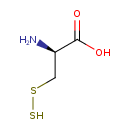
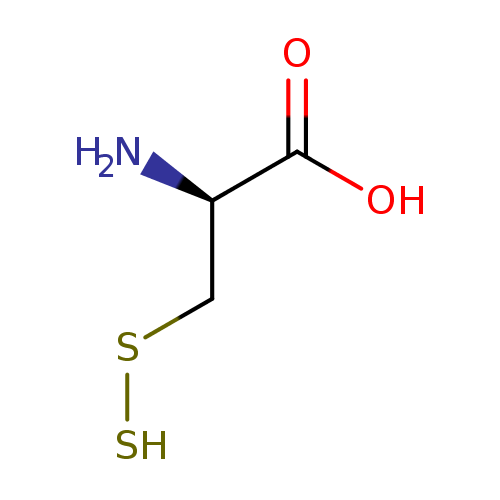
thiocysteine |
|---|
| Description: | An S-substituted L-cysteine where the S-substituent is specified as sulfanyl. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (R)-2-amino-3-disulfanylpropanoic acid
- 2-amino-3-disulfanylpropanoic acid
- 2-amino-3-hydrodisulfidopropanoic acid
- 2-amino-3-hydropersulfidopropanoic acid
- 2-amino-3-persulfhydrylpropanoic acid
- 3-(thiosulfeno)-alanine
- cysteine persulfide
- cysteine perthiol
- S-sulfanylcysteine
- Thiocysteine
- thiocysteine
|
|---|
|
Chemical Formula: |
C3H7NO2S2 |
|---|
| Average Molecular Weight: |
153.214 |
|---|
| Monoisotopic Molecular
Weight: |
153.99965 |
|---|
| InChI Key: |
XBKONSCREBSMCS-REOHCLBHSA-N |
|---|
| InChI: | InChI=1S/C3H7NO2S2/c4-2(1-8-7)3(5)6/h2,7H,1,4H2,(H,5,6)/t2-/m0/s1 |
|---|
| CAS
number: |
5652-32-4 |
|---|
| IUPAC Name: | 3-disulfanyl-L-alanine |
|---|
|
Traditional IUPAC Name: |
(2S)-2-amino-3-disulfanylpropanoic acid |
|---|
| SMILES: | C(SS)C(C([O-])=O)[N+] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as cysteine and derivatives. These are compounds containing cysteine or a derivative thereof resulting from reaction of cysteine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Cysteine and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Cysteine or derivatives
- Alpha-amino acid
- D-alpha-amino acid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Sulfenyl compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -1.917 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Ollagnier-de-Choudens S, Lascoux D, Loiseau L, Barras F, Forest E, Fontecave M (2003)Mechanistic studies of the SufS-SufE cysteine desulfurase: evidence for sulfur transfer from SufS to SufE. FEBS letters 555, Pubmed: 14644425
- Mihara H, Esaki N (2002)Bacterial cysteine desulfurases: their function and mechanisms. Applied microbiology and biotechnology 60, Pubmed: 12382038
- Kaiser JT, Bruno S, Clausen T, Huber R, Schiaretti F, Mozzarelli A, Kessler D (2003)Snapshots of the cystine lyase C-DES during catalysis. Studies in solution and in the crystalline state. The Journal of biological chemistry 278, Pubmed: 12386155
- Kurihara T, Mihara H, Kato S, Yoshimura T, Esaki N (2003)Assembly of iron-sulfur clusters mediated by cysteine desulfurases, IscS, CsdB and CSD, from Escherichia coli. Biochimica et biophysica acta 1647, Pubmed: 12686149
- Clausen T, Kaiser JT, Steegborn C, Huber R, Kessler D (2000)Crystal structure of the cystine C-S lyase from Synechocystis: stabilization of cysteine persulfide for FeS cluster biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 97, Pubmed: 10760256
- Kambampati R, Lauhon CT (2000)Evidence for the transfer of sulfane sulfur from IscS to ThiI during the in vitro biosynthesis of 4-thiouridine in Escherichia coli tRNA. The Journal of biological chemistry 275, Pubmed: 10753862
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|