|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120268 |
|---|
|
Identification |
|---|
| Name: |
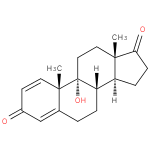
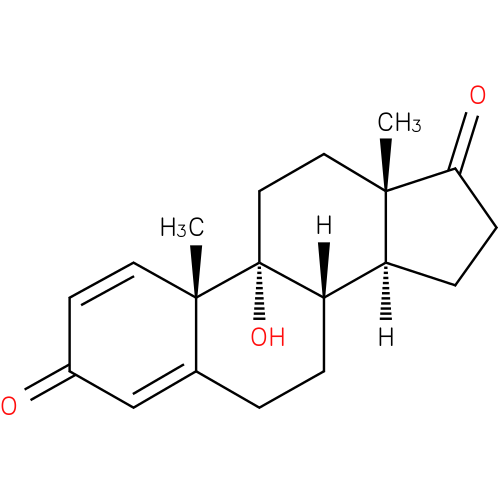
9α-hydroxyandrosta-1,4-diene-3,17-dione |
|---|
| Description: | A steroid that consists of androstane having double bonds at positions 1 and 4, two keto groups at positions 3 and 17 and a hydroxy group at position 9. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 9alpha-Hydroxyandrosta-1,4-diene-3,17-dione
- 9α-hydroxyandrosta-1,4-diene-3,17-dione
|
|---|
|
Chemical Formula: |
C19H24O3 |
|---|
| Average Molecular Weight: |
300.397 |
|---|
| Monoisotopic Molecular
Weight: |
300.17255 |
|---|
| InChI Key: |
JCEUDJXAQHPZGL-PLOWYNNNSA-N |
|---|
| InChI: | InChI=1S/C19H24O3/c1-17-9-10-19(22)15(14(17)5-6-16(17)21)4-3-12-11-13(20)7-8-18(12,19)2/h7-8,11,14-15,22H,3-6,9-10H2,1-2H3/t14-,15-,17-,18-,19+/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 9-hydroxyandrosta-1,4-diene-3,17-dione |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC13(C(=O)CC[CH]1[CH]2(CCC4(C(C)(C2(O)CC3)C=CC(=O)C=4))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
|
Direct Parent |
Androgens and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Androgen-skeleton
- 3-oxo-delta-1,4-steroid
- 3-oxosteroid
- 17-oxosteroid
- Oxosteroid
- Delta-1,4-steroid
- Cyclic alcohol
- Tertiary alcohol
- Ketone
- Cyclic ketone
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic homopolycyclic compounds |
|---|
| External Descriptors |
- a 3-oxo-\u0026Delta;\u003cSUP\u003e4\u003c/SUP\u003e-steroid (CPD-13680)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | 9α-hydroxyandrosta-1,4-diene-3,17-dione → 3-hydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dioneandrosta-1,4-diene-3,17-dione + Oxygen + NADH + Hydrogen ion → 9α-hydroxyandrosta-1,4-diene-3,17-dione + NAD+ + Water |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Petrusma M, Dijkhuizen L, van der Geize R (2009)Rhodococcus rhodochrous DSM 43269 3-ketosteroid 9alpha-hydroxylase, a two-component iron-sulfur-containing monooxygenase with subtle steroid substrate specificity. Applied and environmental microbiology 75, Pubmed: 19561185
- Capyk JK, D'Angelo I, Strynadka NC, Eltis LD (2009)Characterization of 3-ketosteroid 9{alpha}-hydroxylase, a Rieske oxygenase in the cholesterol degradation pathway of Mycobacterium tuberculosis. The Journal of biological chemistry 284, Pubmed: 19234303
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|