|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120246 |
|---|
|
Identification |
|---|
| Name: |
scopoletin |
|---|
| Description: | A hydroxycoumarin that is umbelliferone bearing a methoxy substituent at position 6. |
|---|
|
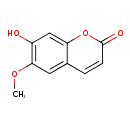
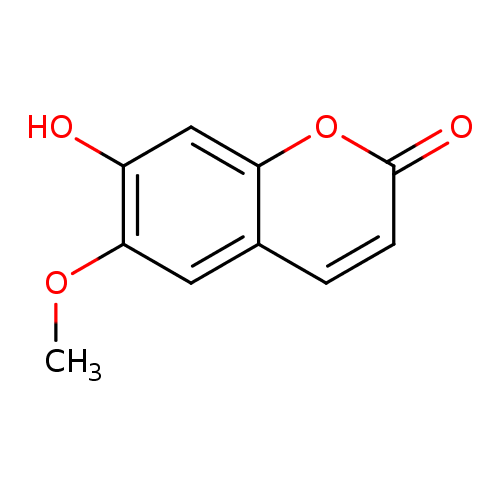
Structure |
|
|---|
| Synonyms: | - 6-Methoxy-7-hydroxycoumarin
- 6-Methylesculetin
- 6-O-Methylesculetin
- 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one
- 7-hydroxy-6-methoxycoumarin
- Scopoletin
- scopoletin
|
|---|
|
Chemical Formula: |
C10H8O4 |
|---|
| Average Molecular Weight: |
192.171 |
|---|
| Monoisotopic Molecular
Weight: |
192.04225 |
|---|
| InChI Key: |
RODXRVNMMDRFIK-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3 |
|---|
| CAS
number: |
92-61-5 |
|---|
| IUPAC Name: | 7-hydroxy-6-methoxy-2H-chromen-2-one |
|---|
|
Traditional IUPAC Name: |
scopoletin |
|---|
| SMILES: | COC2(C=C1(C(OC(=O)C=C1)=CC=2O)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as 7-hydroxycoumarins. These are coumarins that contain one or more hydroxyl groups attached to the C7 position the coumarin skeleton. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
|
Class |
Coumarins and derivatives |
|---|
| Sub Class | Hydroxycoumarins |
|---|
|
Direct Parent |
7-hydroxycoumarins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 7-hydroxycoumarin
- 1-benzopyran
- Methoxyphenol
- Benzopyran
- Anisole
- Pyranone
- Alkyl aryl ether
- Benzenoid
- Pyran
- Heteroaromatic compound
- Lactone
- Oxacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Ether
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
204 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 204 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|