|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120245 |
|---|
|
Identification |
|---|
| Name: |
ethylnitronate |
|---|
| Description: | A nitrogen oxoanion arising from deprotonation of nitroethane. |
|---|
|
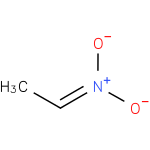
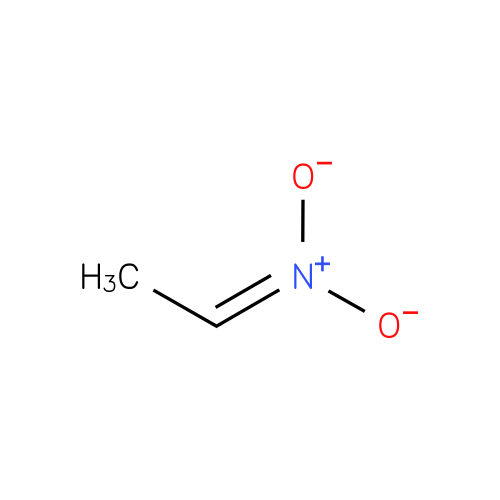
Structure |
|
|---|
| Synonyms: | - aci-Nitroethane ion(1-)
- Ethanenitronate
- Nitroethane aci-anion
- Nitroethane anion
|
|---|
|
Chemical Formula: |
C2H4NO2 |
|---|
| Average Molecular Weight: |
74.059 |
|---|
| Monoisotopic Molecular
Weight: |
75.03203 |
|---|
| InChI Key: |
YERBBVNYIKLXDM-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C2H4NO2/c1-2-3(4)5/h2H,1H3/q-1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [ethylidene(oxido)-λ5-azanyl]oxidanide |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC=N(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as c-nitro compounds. These are compounds having the nitro group, -NO2 (free valence on nitrogen), which is attached to carbon. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic 1,3-dipolar compounds |
|---|
|
Class |
Allyl-type 1,3-dipolar organic compounds |
|---|
| Sub Class | Organic nitro compounds |
|---|
|
Direct Parent |
C-nitro compounds |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- C-nitro compound
- Organic oxoazanium
- Organic nitrogen compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
- nitrogen oxoanion (CHEBI:55327)
- a small molecule (CPD-320)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Gadda G, Francis K (2010)Nitronate monooxygenase, a model for anionic flavin semiquinone intermediates in oxidative catalysis. Archives of biochemistry and biophysics 493, Pubmed: 19577534
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|