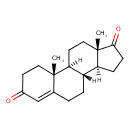
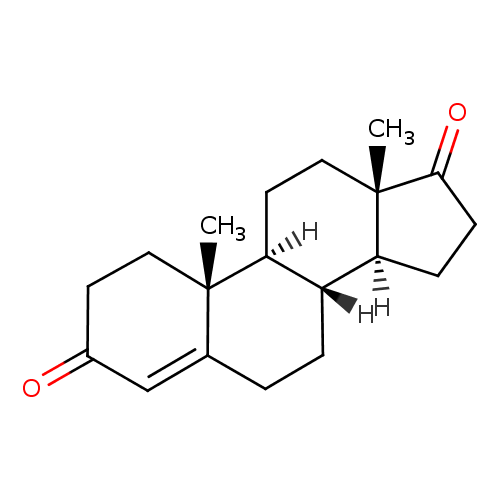
| References: |
- Berkovitz GD, Guerami A, Brown TR, MacDonald PC, Migeon CJ: Familial gynecomastia with increased extraglandular aromatization of plasma carbon19-steroids. J Clin Invest. 1985 Jun;75(6):1763-9. [3924954 ]
- Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Pizzorni C, Paolino S, Seriolo B, Felli L, Straub RH: Anti-TNF and sex hormones. Ann N Y Acad Sci. 2006 Jun;1069:391-400. [16855166 ]
- Schwarz S, Pohl P: Steroid hormones and steroid hormone binding globulins in cerebrospinal fluid studied in individuals with intact and with disturbed blood-cerebrospinal fluid barrier. Neuroendocrinology. 1992 Feb;55(2):174-82. [1620285 ]
- van Vloten WA, van Haselen CW, van Zuuren EJ, Gerlinger C, Heithecker R: The effect of 2 combined oral Contraceptives containing either drospirenone or cyproterone acetate on acne and seborrhea. Cutis. 2002 Apr;69(4 Suppl):2-15. [12096825 ]
- Jasuja R, Ramaraj P, Mac RP, Singh AB, Storer TW, Artaza J, Miller A, Singh R, Taylor WE, Lee ML, Davidson T, Sinha-Hikim I, Gonzalez-Cadavid N, Bhasin S: Delta-4-androstene-3,17-dione binds androgen receptor, promotes myogenesis in vitro, and increases serum testosterone levels, fat-free mass, and muscle strength in hypogonadal men. J Clin Endocrinol Metab. 2005 Feb;90(2):855-63. Epub 2004 Nov 2. [15522925 ]
- Egloff M, Savoure N, Tardivel-Lacombe J, Massart C, Nicol M, Degrelle H: Influence of sex hormone binding globulin and serum albumin on the conversion of androstenedione to testosterone by human erythrocytes. Acta Endocrinol (Copenh). 1981 Jan;96(1):136-40. [7192922 ]
- Zarrilli S, Lombardi G, Paesano L, Di Somma C, Colao A, Mirone V, De Rosa M: Hormonal and seminal evaluation of Leydig cell tumour patients before and after orchiectomy. Andrologia. 2000 May;32(3):147-54. [10863969 ]
- Heikkila R, Aho K, Heliovaara M, Hakama M, Marniemi J, Reunanen A, Knekt P: Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: a longitudinal study. Cancer. 1999 Jul 15;86(2):312-5. [10421267 ]
- Schlaghecke R, Kley HK, Kruskemper HL: The measurement of 4-androstene-3, 11, 17-trione (11-oxo-androstenedione) by radioimmunoassay in human plasma. Steroids. 1984 Jul;44(1):23-33. [6100340 ]
- King DS, Sharp RL, Vukovich MD, Brown GA, Reifenrath TA, Uhl NL, Parsons KA: Effect of oral androstenedione on serum testosterone and adaptations to resistance training in young men: a randomized controlled trial. JAMA. 1999 Jun 2;281(21):2020-8. [10359391 ]
- Johansson A, Henriksson A, Olofsson BO, Olsson T: Adrenal steroid dysregulation in dystrophia myotonica. J Intern Med. 1999 Apr;245(4):345-51. [10356596 ]
- Atkinson G, Campbell DJ, Cawood ML, Oakey RE: Steroids in human intrauterine fluids of early pregnancy. Clin Endocrinol (Oxf). 1996 Apr;44(4):435-40. [8706310 ]
- Zarrilli S, Paesano L, Mirone V, Finelli L, De Rosa M: [Evaluation of seminal and hormonal parameters in idiopathic varicocele before and after surgical intervention] Chir Ital. 1998 Mar-Aug;50(2-4):21-8. [11762080 ]
- Berria R, Gastaldelli A, Lucidi S, Belfort R, De Filippis E, Easton C, Brytzki R, Cusi K, Jovanovic L, DeFronzo R: Reduction in hematocrit level after pioglitazone treatment is correlated with decreased plasma free testosterone level, not hemodilution, in women with polycystic ovary syndrome. Clin Pharmacol Ther. 2006 Aug;80(2):105-14. Epub 2006 Jun 30. [16890572 ]
- Thomson S, Wallace AM, Cook B: A 125I-radioimmunoassay for measuring androstenedione in serum and in blood-spot samples from neonates. Clin Chem. 1989 Aug;35(8):1706-12. [2758640 ]
- Riikonen RS: How do cryptogenic and symptomatic infantile spasms differ? Review of biochemical studies in Finnish patients. J Child Neurol. 1996 Sep;11(5):383-8. [8877606 ]
- Azurmendi A, Braza F, Sorozabal A, Garcia A, Braza P, Carreras MR, Munoz JM, Cardas J, Sanchez-Martin JR: Cognitive abilities, androgen levels, and body mass index in 5-year-old children. Horm Behav. 2005 Aug;48(2):187-95. [15878571 ]
- Schairer C, Hill D, Sturgeon SR, Fears T, Mies C, Ziegler RG, Hoover RN, Sherman ME: Serum concentrations of estrogens, sex hormone binding globulin, and androgens and risk of breast hyperplasia in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005 Jul;14(7):1660-5. [16030098 ]
- Feldman PS, Kovacs K, Horvath E, Adelson GL: Malignant Leydig cell tumor: clinical, histologic and electron microscopic features. Cancer. 1982 Feb 15;49(4):714-21. [7055782 ]
|
|---|