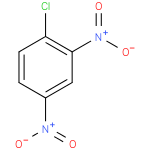
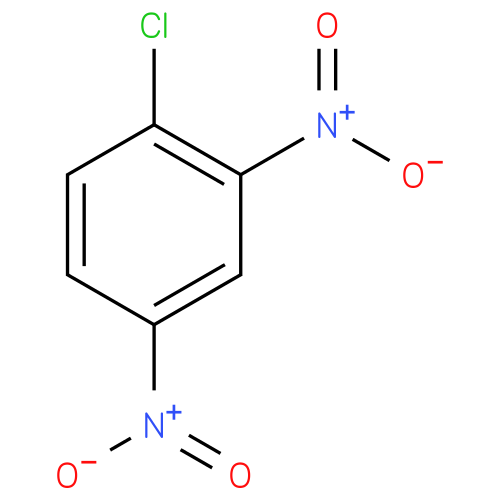
| Synonyms: | - 1,3-Dinitro-4-chlorobenzene
- 1-chloro-2,4-dinitrobenzene
- 1-Chloro-2,4-dinitrobenzene
- 1-Chloro-2,4-dinitrobenzol
- 2,4-Dinitro-1-chlorobenzene
- 2,4-Dinitrochlorobenzene
- 2,4-Dinitrophenyl chloride
- 4-Chloro-1,3-dinitrobenzene
- 6-Chloro-1,3-dinitrobenzene
- Cdnb
- Chlorodinitrobenzene
- ClDNB
- Dinitrochlorobenzene
- Dncb
- DNCB
- DNPCl
|
|---|
| References: |
- Maurer T, Thomann P, Weirich EG, Hess R (1975)The optimization test in the guinea-pig. A method for the predictive evaluation of the contact allergenicity of chemicals. Agents and actions 5, Pubmed: 1155304
- Palmer RA, Friedmann PS (2004)Ultraviolet radiation causes less immunosuppression in patients with polymorphic light eruption than in controls. The Journal of investigative dermatology 122, Pubmed: 15009707
- Dearman RJ, Basketter DA, Kimber I (1992)Variable effects of chemical allergens on serum IgE concentration in mice. Preliminary evaluation of a novel approach to the identification of respiratory sensitizers. Journal of applied toxicology : JAT 12, Pubmed: 1447476
- Kato H, Hayashi M, Fukumori Y, Kaneko H (2002)MHC restriction in contact hypersensitivity to dicyclohexylcarbodiimide. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 40, Pubmed: 12176098
- Miyauchi H, Horio T (1992)A new animal model for contact dermatitis: the hairless guinea pig. The Journal of dermatology 19, Pubmed: 1640019
- Kuper CF, Stierum RH, Boorsma A, Schijf MA, Prinsen M, Bruijntjes JP, Bloksma N, Arts JH (2008)The contact allergen dinitrochlorobenzene (DNCB) and respiratory allergy in the Th2-prone Brown Norway rat. Toxicology 246, Pubmed: 18316151
- Ju C (2009)The role of haptic macrophages in regulation of idiosyncratic drug reactions. Toxicologic pathology 37, Pubmed: 19171927
- Hopkins JE, Naisbitt DJ, Humphreys N, Dearman RJ, Kimber I, Park BK (2005)Exposure of mice to the nitroso metabolite of sulfamethoxazole stimulates interleukin 5 production by CD4+ T-cells. Toxicology 206, Pubmed: 15588915
- Henjakovic M, Martin C, Hoymann HG, Sewald K, Ressmeyer AR, Dassow C, Pohlmann G, Krug N, Uhlig S, Braun A (2008)Ex vivo lung function measurements in precision-cut lung slices (PCLS) from chemical allergen-sensitized mice represent a suitable alternative to in vivo studies. Toxicological sciences : an official journal of the Society of Toxicology 106, Pubmed: 18775882
- Kuroda Y, Takashima H (1990)Impairment of cell-mediated immune responses in HTLV-I-associated myelopathy. Journal of the neurological sciences 100, Pubmed: 1982449
- Burch WM, Snyderman R (1982)Induction of cellular immunity to Coccidioides immitis after sensitization with dinitrochlorobenzene. Annals of internal medicine 96, Pubmed: 7059096
- Takanami I, Nakayama H (1988)TMCHB: a possible alternative to DNCB in skin testing for immune competence. Contact dermatitis 19, Pubmed: 3180787
- Slovak AJ (1980)Contact dermatitis due to benzisothiazolone in a works analytical team. Contact dermatitis 6, Pubmed: 6446435
- Sato T, Maguire HC, Mastrangelo MJ, Berd D (1995)Human immune response to DNP-modified autologous cells after treatment with a DNP-conjugated melanoma vaccine. Clinical immunology and immunopathology 74, Pubmed: 7994925
- Schultz KT, Maguire HC (1982)Chemically-induced delayed hypersensitivity in the cat. Veterinary immunology and immunopathology 3, Pubmed: 7179722
- Nunomura S, Ohtsubo-Yoshioka M, Okayama Y, Terui T, Ra C (2015)FcR? promotes contact hypersensitivity to oxazolone without affecting the contact sensitisation process in B6 mice. Experimental dermatology 24, Pubmed: 25515858
|
|---|