|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120182 |
|---|
|
Identification |
|---|
| Name: |
phosphocholine |
|---|
| Description: | The organophosphate oxoanion formed from choline by removal of two protons from the phosphate group. Major species at pH 7.3. |
|---|
|
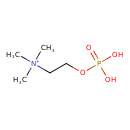
Structure |
|
|---|
| Synonyms: | - 2-(trimethylammonio)ethyl phosphate
- phosphocholine
|
|---|
|
Chemical Formula: |
C5H13NO4P |
|---|
| Average Molecular Weight: |
182.136 |
|---|
| Monoisotopic Molecular
Weight: |
184.07387 |
|---|
| InChI Key: |
YHHSONZFOIEMCP-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C5H14NO4P/c1-6(2,3)4-5-10-11(7,8)9/h4-5H2,1-3H3,(H-,7,8,9)/p-1 |
|---|
| CAS
number: |
3616-04-4 |
|---|
| IUPAC Name: | 2-(trimethylazaniumyl)ethyl phosphate |
|---|
|
Traditional IUPAC Name: |
ChoP |
|---|
| SMILES: | C[N+](CCOP([O-])([O-])=O)(C)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as phosphocholines. These are compounds containing a [2-(trimethylazaniumyl)ethoxy]phosphonic acid or derivative. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic nitrogen compounds |
|---|
| Sub Class | Organonitrogen compounds |
|---|
|
Direct Parent |
Phosphocholines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Phosphocholine
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Tetraalkylammonium salt
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Organooxygen compound
- Amine
- Organic cation
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-0900000000-3d961174f1a27a76e351 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-9300000000-fff62078da4bf8f84753 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-007a-9000000000-275c66ca16bdb9344ee2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-001i-0900000000-1dbd5123486fc544909b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-001r-4900000000-a77ea15e15088c9209fe | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-002r-9600000000-55422f402ca1581e8111 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0072-9100000000-80e4e45c1a0a5ef016ce | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-006t-9000000000-347eed4168ee48195753 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0040-6900000000-8bbb64f5a93a59787a0d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-001i-9700000000-1e35f21857e363fec75a | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Calas M, Cordina G, Bompart J, Ben Bari M, Jei T, Ancelin ML, Vial H (1997)Antimalarial activity of molecules interfering with Plasmodium falciparum phospholipid metabolism. Structure-activity relationship analysis. Journal of medicinal chemistry 40, Pubmed: 9357523
|
|---|
| Synthesis Reference: |
Vijeeta, T.; Reddy, J. R. C.; Rao, B. V. S. K.; Karuna, M. S. L.; Prasad, R. B. N. Phospholipase-mediated preparation of 1-ricinoleoyl-2-acyl-sn- glycero-3-phosphocholine from soya and egg phosphatidylcholine. Biotechnology Letters (2004), 26(13), 1077-10 |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|