|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120153 |
|---|
|
Identification |
|---|
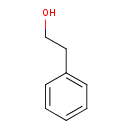
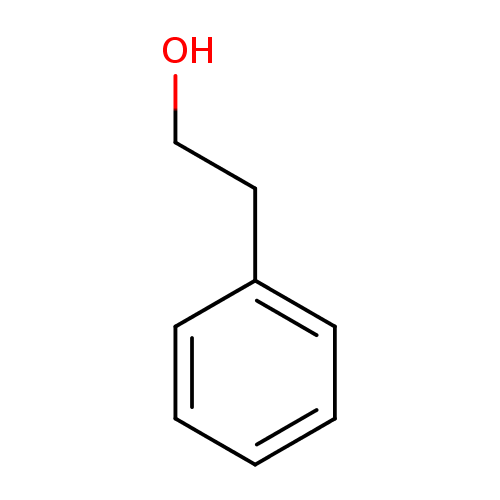
| Name: |
2-phenylethanol |
|---|
| Description: | A primary alcohol that is ethanol substituted by a phenyl group at position 2. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-Hydroxyethylbenzene
- 2-PEA
- 2-PHENYL-ETHANOL
- 2-Phenylethanol
- Benzeneethanol
- Benzylmethanol
- β-PEA
- β-Phenethyl alcohol
- β-Phenylethanol
- β-Phenylethyl alcohol
- Phenethyl alcohol
- Phenylethyl alcohol
|
|---|
|
Chemical Formula: |
C8H10O |
|---|
| Average Molecular Weight: |
122.166 |
|---|
| Monoisotopic Molecular
Weight: |
122.073166 |
|---|
| InChI Key: |
WRMNZCZEMHIOCP-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C8H10O/c9-7-6-8-4-2-1-3-5-8/h1-5,9H,6-7H2 |
|---|
| CAS
number: |
60-12-8 |
|---|
| IUPAC Name: | 2-phenylethanol |
|---|
|
Traditional IUPAC Name: |
phenylethanol |
|---|
| SMILES: | C1(C=CC(CCO)=CC=1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as benzene and substituted derivatives. These are aromatic compounds containing one monocyclic ring system consisting of benzene. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Benzene and substituted derivatives |
|---|
|
Direct Parent |
Benzene and substituted derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
-25.8 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | -25.8 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 22.2 mg/mL at 25 °C | Not Available | | LogP | 1.36 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Politano VT, Diener RM, Christian MS, Hoberman AM, Palmer A, Ritacco G, Adams TB, Api AM (2013)Oral and dermal developmental toxicity studies of phenylethyl alcohol in rats. International journal of toxicology 32, Pubmed: 23385159
- Farag MA, Al-Mahdy DA (2013)Comparative study of the chemical composition and biological activities of Magnolia grandiflora and Magnolia virginiana flower essential oils. Natural product research 27, Pubmed: 22690913
- Xiao L, Lee J, Zhang G, Ebeler SE, Wickramasinghe N, Seiber J, Mitchell AE (2014)HS-SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis). Food chemistry 151, Pubmed: 24423498
- Kim B, Cho BR, Hahn JS (2014)Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnology and bioengineering 111, Pubmed: 23836015
- Gao F, Daugulis AJ (2009)Bioproduction of the aroma compound 2-phenylethanol in a solid-liquid two-phase partitioning bioreactor system by Kluyveromyces marxianus. Biotechnology and bioengineering 104, Pubmed: 19517523
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|