|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120131 |
|---|
|
Identification |
|---|
| Name: |
indole-3-acetamide |
|---|
| Description: | A member of the class of indoles that is acetamide substituted by a 1H-indol-3-yl group at position 2. It is an intermediate in the production of plant hormone indole acetic acid (IAA). |
|---|
|
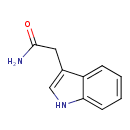
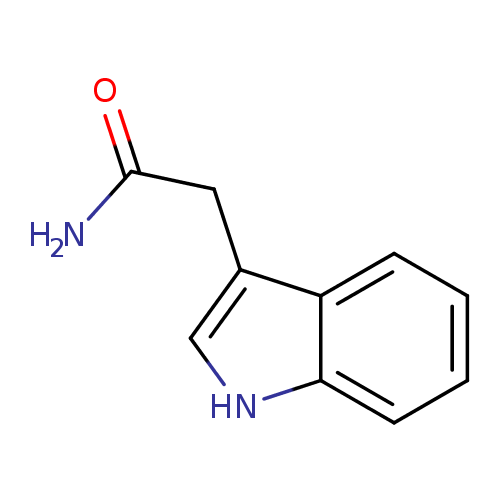
Structure |
|
|---|
| Synonyms: | - 1H-indole-3-acetamide
- 3-indoleacetamide
- Indole-3-acetamide
- indole-3-acetamide
- Indole-3-acetamide
- indoleacetamide
|
|---|
|
Chemical Formula: |
C10H10N2O |
|---|
| Average Molecular Weight: |
174.202 |
|---|
| Monoisotopic Molecular
Weight: |
174.07932 |
|---|
| InChI Key: |
ZOAMBXDOGPRZLP-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C10H10N2O/c11-10(13)5-7-6-12-9-4-2-1-3-8(7)9/h1-4,6,12H,5H2,(H2,11,13) |
|---|
| CAS
number: |
879-37-8 |
|---|
| IUPAC Name: | 2-(1H-indol-3-yl)acetamide |
|---|
|
Traditional IUPAC Name: |
indole-3-acetamide |
|---|
| SMILES: | C1(=C(CC(N)=O)C2(=CC=CC=C(N1)2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 3-alkylindoles. These are compounds containing an indole moiety that carries an alkyl chain at the 3-position. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Indoles and derivatives |
|---|
|
Direct Parent |
3-alkylindoles |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 3-alkylindole
- Benzenoid
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Carboximidic acid derivative
- Carboximidic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
150 - 151 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 150 - 151 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- indole-3-acetate biosynthesis IV (bacteria)PWY-5025
- indole-3-acetate biosynthesis III (bacteria)PWY-3161
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Yin C, Park JJ, Gang DR, Hulbert SH (2014)Characterization of a tryptophan 2-monooxygenase gene from Puccinia graminis f. sp. tritici involved in auxin biosynthesis and rust pathogenicity. Molecular plant-microbe interactions : MPMI 27, Pubmed: 24350783
- Gaweska HM, Taylor AB, Hart PJ, Fitzpatrick PF (2013)Structure of the flavoprotein tryptophan 2-monooxygenase, a key enzyme in the formation of galls in plants. Biochemistry 52, Pubmed: 23521653
- Dimkpa CO, Zeng J, McLean JE, Britt DW, Zhan J, Anderson AJ (2012)Production of indole-3-acetic acid via the indole-3-acetamide pathway in the plant-beneficial bacterium Pseudomonas chlororaphis O6 is inhibited by ZnO nanoparticles but enhanced by CuO nanoparticles. Applied and environmental microbiology 78, Pubmed: 22210218
- Kulkarni GB, Sanjeevkumar S, Kirankumar B, Santoshkumar M, Karegoudar TB (2013)Indole-3-acetic acid biosynthesis in Fusarium delphinoides strain GPK, a causal agent of Wilt in Chickpea. Applied biochemistry and biotechnology 169, Pubmed: 23306880
- Tsavkelova E, Oeser B, Oren-Young L, Israeli M, Sasson Y, Tudzynski B, Sharon A (2012)Identification and functional characterization of indole-3-acetamide-mediated IAA biosynthesis in plant-associated Fusarium species. Fungal genetics and biology : FG & B 49, Pubmed: 22079545
- Mano Y, Nemoto K (2012)The pathway of auxin biosynthesis in plants. Journal of experimental botany 63, Pubmed: 22447967
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|