|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120120 |
|---|
|
Identification |
|---|
| Name: |
2-oxovalerate |
|---|
| Description: | A 2-oxo monocarboxylic acid anion that is the conjugate base of 2-oxopentanoic acid, obtained by deprotonation of the carboxy group. |
|---|
|
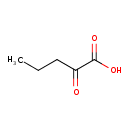
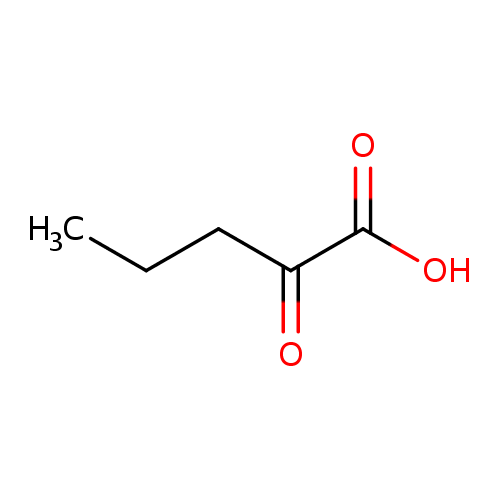
Structure |
|
|---|
| Synonyms: | - 2-Oxopentanoate
- 2-oxopentanoate
- 2-oxovalerate
- α-ketovalerate
|
|---|
|
Chemical Formula: |
C5H7O3 |
|---|
| Average Molecular Weight: |
115.108 |
|---|
| Monoisotopic Molecular
Weight: |
116.04734 |
|---|
| InChI Key: |
KDVFRMMRZOCFLS-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C5H8O3/c1-2-3-4(6)5(7)8/h2-3H2,1H3,(H,7,8)/p-1 |
|---|
| CAS
number: |
1821-02-9 |
|---|
| IUPAC Name: | 2-oxopentanoate |
|---|
|
Traditional IUPAC Name: |
?-oxopentanoic acid |
|---|
| SMILES: | CCCC(=O)C(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Keto acids and derivatives |
|---|
|
Direct Parent |
Short-chain keto acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Branched fatty acid
- Methyl-branched fatty acid
- Short-chain keto acid
- Alpha-keto acid
- Fatty acyl
- Alpha-hydroxy ketone
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
6.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 6.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 0.35 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Lee SH, Kim SO, Chung BC: Gas chromatographic-mass spectrometric determination of urinary oxoacids using O-(2,3,4,5,6-pentafluorobenzyl)oxime-trimethylsilyl ester derivatization and cation-exchange chromatography. J Chromatogr B Biomed Sci Appl. 1998 Nov 20;719(1-2):1-7. [9869358 ]
- Fu X, Kimura M, Iga M, Yamaguchi S: Gas chromatographic-mass spectrometric screening for organic acidemias using dried urine filter paper: determination of alpha-ketoacids. J Chromatogr B Biomed Sci Appl. 2001 Jul 5;758(1):87-94. [11482739 ]
- Wang ZJ, Zaitsu K, Ohkura Y: High-performance liquid chromatographic determination of alpha-keto acids in human serum and urine using 1,2-diamino-4,5-methylenedioxybenzene as a precolumn fluorescence derivatization reagent. J Chromatogr. 1988 Sep 9;430(2):223-31. [3235498 ]
|
|---|
| Synthesis Reference: |
Lieberman, Irving; Barker, H. A. b-Keto acid formation and decomposition by preparations of Clostridium kluyveri. Journal of Bacteriology (1954), 68 329-33. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|