|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120102 |
|---|
|
Identification |
|---|
| Name: |
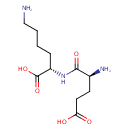
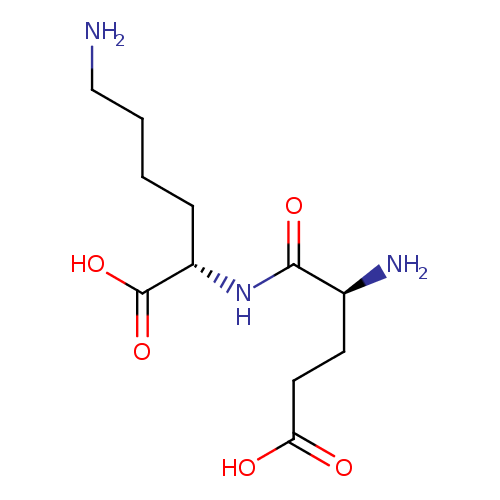
UDP-N-acetylmuramoyl-L-alanyl-γ-D-glutamyl-L-lysine |
|---|
| Description: | Glutamyl-L-lysine is a dipeptide whose absorption is facilitated by the human intestinal oligopeptide transporter (PEPT1) (PMID 16759105 ). Glutamyl-L-lysine is an isopeptides found in human hair. ( Schriftenreihe des Deutschen Wollforschungsinstitutes (Technische Hochschule Aachen) (1979), (Sonderband, Haarwiss. Symp. Dtsch. Wollforschungsinst., 1st, 1978), 128-30. ). |
|---|
|
Structure |
|
|---|
| Synonyms: | - UDP-N-acetylmuramoyl-L-alanyl-alpha-D-glutamyl-L-lysine
- UDP-N-acetylmuramoyl-L-alanyl-D-glutamyl-L-lysine
|
|---|
|
Chemical Formula: |
C34H52N7O24P2 |
|---|
| Average Molecular Weight: |
1004.765 |
|---|
| Monoisotopic Molecular
Weight: |
1008.2852 |
|---|
| InChI Key: |
WXBLSQNZKMJACT-BYEZXYKXSA-K |
|---|
| InChI: | InChI=1S/C34H55N7O24P2/c1-14(28(49)39-18(32(53)54)7-8-21(44)38-17(31(51)52)6-4-5-10-35)36-29(50)15(2)61-27-23(37-16(3)43)33(63-19(12-42)25(27)47)64-67(58,59)65-66(56,57)60-13-20-24(46)26(48)30(62-20)41-11-9-22(45)40-34(41)55/h9,11,14-15,17-20,23-27,30,33,42,46-48H,4-8,10,12-13,35H2,1-3H3,(H,36,50)(H,37,43)(H,38,44)(H,39,49)(H,51,52)(H,53,54)(H,56,57)(H,58,59)(H,40,45,55)/p-3/t14-,15+,17-,18+,19+,20+,23+,24+,25+,26+,27+,30+,33+/m0/s1 |
|---|
| CAS
number: |
5891-53-2 |
|---|
| IUPAC Name: | (2S)-6-amino-2-[(2S)-2-amino-4-carboxybutanamido]hexanoic acid |
|---|
|
Traditional IUPAC Name: |
L-?-glutamyl-L-lysine |
|---|
| SMILES: | CC(NC(=O)C(C)OC3(C(O)C(CO)OC(OP(=O)([O-])OP(=O)([O-])OCC1(OC(C(O)C(O)1)N2(C=CC(=O)NC(=O)2)))C(NC(C)=O)3))C(=O)NC(CCC(NC(CCCC[N+])C(=O)[O-])=O)C([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Dipeptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-dipeptide
- Glutamic acid or derivatives
- N-acyl-l-alpha-amino acid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Medium-chain fatty acid
- Amino fatty acid
- Dicarboxylic acid or derivatives
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Fatty acid
- Amino acid or derivatives
- Carboxamide group
- Carboxylic acid salt
- Amino acid
- Secondary carboxylic acid amide
- Carboxylic acid
- Organic salt
- Hydrocarbon derivative
- Primary aliphatic amine
- Amine
- Organic oxide
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Organopnictogen compound
- Organic zwitterion
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Jeitner TM, Bogdanov MB, Matson WR, Daikhin Y, Yudkoff M, Folk JE, Steinman L, Browne SE, Beal MF, Blass JP, Cooper AJ: N(epsilon)-(gamma-L-glutamyl)-L-lysine (GGEL) is increased in cerebrospinal fluid of patients with Huntington's disease. J Neurochem. 2001 Dec;79(5):1109-12. [11739625 ]
- Vig BS, Stouch TR, Timoszyk JK, Quan Y, Wall DA, Smith RL, Faria TN: Human PEPT1 pharmacophore distinguishes between dipeptide transport and binding. J Med Chem. 2006 Jun 15;49(12):3636-44. [16759105 ]
|
|---|
| Synthesis Reference: |
Khosla, M. C.; Anand, Nitya. Synthesis of Na-(a-glutamyl and aspartyl)lysines. Journal of Scientific and Industrial Research, Section B: Physical Sciences (1962), 21B 287-9. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|