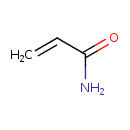
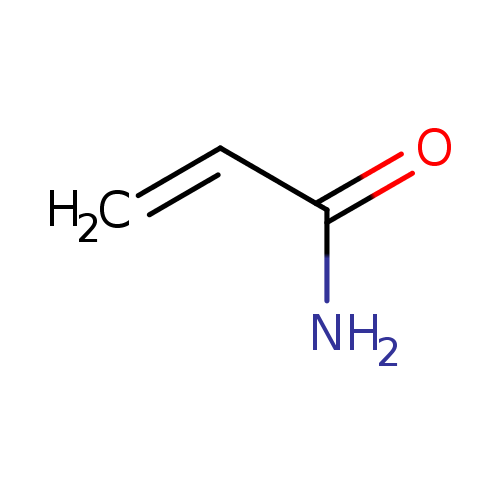
| References: |
- Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M (2002)Analysis of acrylamide, a carcinogen formed in heated foodstuffs. Journal of agricultural and food chemistry 50, Pubmed: 12166997
- Adler ID, Baumgartner A, Gonda H, Friedman MA, Skerhut M (2000)1-Aminobenzotriazole inhibits acrylamide-induced dominant lethal effects in spermatids of male mice. Mutagenesis 15, Pubmed: 10719038
- Lopachin RM, Decaprio AP (2005)Protein adduct formation as a molecular mechanism in neurotoxicity. Toxicological sciences : an official journal of the Society of Toxicology 86, Pubmed: 15901921
- Besaratinia A, Pfeifer GP (2004)Genotoxicity of acrylamide and glycidamide. Journal of the National Cancer Institute 96, Pubmed: 15240786
- Fan X, Mastovska K (2006)Effectiveness of ionizing radiation in reducing furan and acrylamide levels in foods. Journal of agricultural and food chemistry 54, Pubmed: 17032038
- Turko IV, Sechi S (2007)Acrylamide--a cysteine alkylating reagent for quantitative proteomics. Methods in molecular biology (Clifton, N.J.) 359, Pubmed: 17484107
- Besaratinia A, Pfeifer GP (2007)A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 28, Pubmed: 17234719
- Zhang Y, Zhang Y (2007)Formation and reduction of acrylamide in Maillard reaction: a review based on the current state of knowledge. Critical reviews in food science and nutrition 47, Pubmed: 17558658
- Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2008)Dietary acrylamide intake and the risk of renal cell, bladder, and prostate cancer. The American journal of clinical nutrition 87, Pubmed: 18469268
- Ghanayem BI, Bai R, Burka LT (2009)Effect of dose volume on the toxicokinetics of acrylamide and its metabolites and 2-deoxy-D-glucose. Drug metabolism and disposition: the biological fate of chemicals 37, Pubmed: 19022940
- Lineback DR, Coughlin JR, Stadler RH (2012)Acrylamide in foods: a review of the science and future considerations. Annual review of food science and technology 3, Pubmed: 22136129
- Garey J, Paule MG (2010)Effects of chronic oral acrylamide exposure on incremental repeated acquisition (learning) task performance in Fischer 344 rats. Neurotoxicology and teratology 32, Pubmed: 19846048
- Haase NU, Grothe KH, Matthäus B, Vosmann K, Lindhauer MG (2012)Acrylamide formation and antioxidant level in biscuits related to recipe and baking. Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment 29, Pubmed: 22784192
- Segerbäck D, Calleman CJ, Schroeder JL, Costa LG, Faustman EM (1995)Formation of N-7-(2-carbamoyl-2-hydroxyethyl)guanine in DNA of the mouse and the rat following intraperitoneal administration of [14C]acrylamide. Carcinogenesis 16, Pubmed: 7767980
- Garey J, Paule MG (2007)Effects of chronic low-dose acrylamide exposure on progressive ratio performance in adolescent rats. Neurotoxicology 28, Pubmed: 17720246
|
|---|