|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120064 |
|---|
|
Identification |
|---|
| Name: |
L-dehydro-ascorbate |
|---|
| Description: | Conjugate base of dehydroascorbic acid arising from removal of the acidic proton at the C-2 position; major species at pH 7.3. |
|---|
|
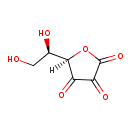
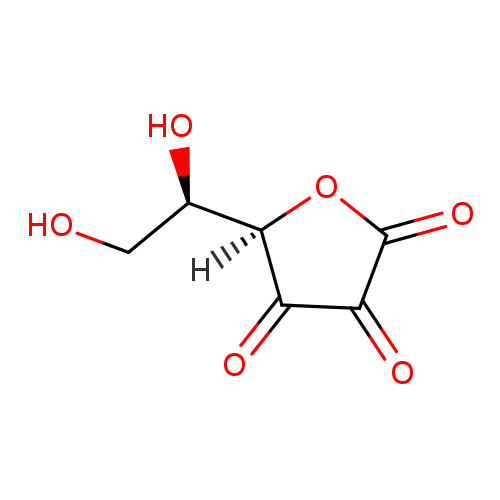
Structure |
|
|---|
| Synonyms: | - 2-(1,2-dihydroxyethyl)-3,4,5-trioxotetrahydrofuran-2-ide
- dehydroascorbic acid anion
- dehydroascorbide anion
|
|---|
|
Chemical Formula: |
C6H6O6 |
|---|
| Average Molecular Weight: |
174.11 |
|---|
| Monoisotopic Molecular
Weight: |
174.01643 |
|---|
| InChI Key: |
SBJKKFFYIZUCET-SZSCBOSDSA-N |
|---|
| InChI: | InChI=1S/C6H6O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-8H,1H2/t2-,5?/m0/s1 |
|---|
| CAS
number: |
490-83-5 |
|---|
| IUPAC Name: | 2-(1,2-dihydroxyethyl)-3,4,5-trioxooxolan-2-ide |
|---|
|
Traditional IUPAC Name: |
(5R)-5-[(1R)-1,2-dihydroxyethyl]oxolane-2,3,4-trione |
|---|
| SMILES: | C(O)C(O)C1(C(=O)C(=O)C(=O)O1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as gamma butyrolactones. These are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Lactones |
|---|
|
Direct Parent |
Gamma butyrolactones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 3-furanone
- Gamma butyrolactone
- Oxolane
- 1,2-diol
- Carboxylic acid ester
- Cyclic ketone
- Secondary alcohol
- Ketone
- Carboxylic acid derivative
- Oxacycle
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Primary alcohol
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Raghavan SA, Sharma P, Dikshit M: Role of ascorbic acid in the modulation of inhibition of platelet aggregation by polymorphonuclear leukocytes. Thromb Res. 2003 May 1;110(2-3):117-26. [12893026 ]
- Kuo SM, Tan D, Boyer JC: Cellular vitamin C accumulation in the presence of copper. Biol Trace Elem Res. 2004 Aug;100(2):125-36. [15326362 ]
- Bakaev VV, Duntau AP: Ascorbic acid in blood serum of patients with pulmonary tuberculosis and pneumonia. Int J Tuberc Lung Dis. 2004 Feb;8(2):263-6. [15139458 ]
- Toivola DM, Isomaa B: Effects of dehydroabietic acid on the erythrocyte membrane. Chem Biol Interact. 1991;79(1):65-78. [2060038 ]
- Dhariwal KR, Hartzell WO, Levine M: Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am J Clin Nutr. 1991 Oct;54(4):712-6. [1897478 ]
- Trepanier LA, Yoder AR, Bajad S, Beckwith MD, Bellehumeur JL, Graziano FM: Plasma ascorbate deficiency is associated with impaired reduction of sulfamethoxazole-nitroso in HIV infection. J Acquir Immune Defic Syndr. 2004 Aug 15;36(5):1041-50. [15247557 ]
- Mendiratta S, Qu ZC, May JM: Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic Biol Med. 1998 Mar 15;24(5):789-97. [9586809 ]
- Padilla CA, Spyrou G, Holmgren A: High-level expression of fully active human glutaredoxin (thioltransferase) in E. coli and characterization of Cys7 to Ser mutant protein. FEBS Lett. 1996 Jan 2;378(1):69-73. [8549805 ]
- Shugalei IuS, Degtiar VV, Butvin IN, Grivenko GP: [Effect of alcohol intoxication on ascorbic and dehydroascorbic acid levels in rat tissue. and human blood] Ukr Biokhim Zh. 1986 May-Jun;58(3):81-3. [3727042 ]
- Bakaev VV, Efremov AV, Tityaev II: Low levels of dehydroascorbic acid in uraemic serum and the partial correction of dehydroascorbic acid deficiency by haemodialysis. Nephrol Dial Transplant. 1999 Jun;14(6):1472-4. [10383010 ]
- Margolis SA, Ziegler RG, Helzlsouer KJ: Ascorbic and dehydroascorbic acid measurement in human serum and plasma. Am J Clin Nutr. 1991 Dec;54(6 Suppl):1315S-1318S. [1962589 ]
- Davis JL Jr, Mendiratta S, May JM: Similarities in the metabolism of alloxan and dehydroascorbate in human erythrocytes. Biochem Pharmacol. 1998 Apr 15;55(8):1301-7. [9719486 ]
- Wells WW, Xu DP, Yang YF, Rocque PA: Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem. 1990 Sep 15;265(26):15361-4. [2394726 ]
- Dubey SS, Palodhi GR, Jain AK: Ascorbic acid, dehydroascorbic acid and glutathione in liver disease. Indian J Physiol Pharmacol. 1987 Oct-Dec;31(4):279-83. [3450633 ]
- May JM, Qu ZC, Whitesell RR, Cobb CE: Ascorbate recycling in human erythrocytes: role of GSH in reducing dehydroascorbate. Free Radic Biol Med. 1996;20(4):543-51. [8904295 ]
|
|---|
| Synthesis Reference: |
Utsumi, Isamu; Harada, Kiyoshi; Miura, Hiroshi. Dehydroascorbic acid. Jpn. Tokkyo Koho (1972), 2 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|