|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120058 |
|---|
|
Identification |
|---|
| Name: |
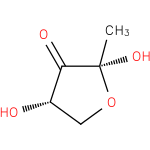
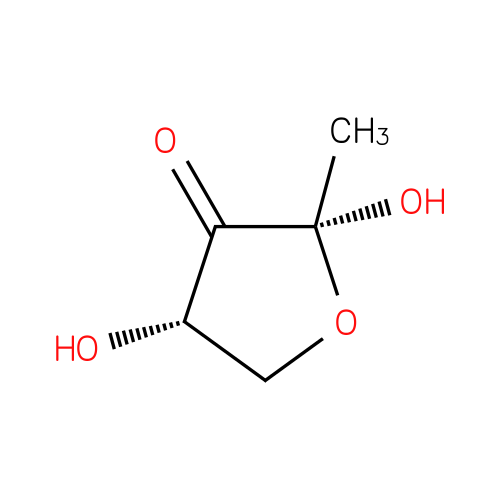
(2R,4S)-2-methyl-2,4-dihydroxydihydrofuran-3-one |
|---|
| Description: | A tetrahydrofuranone that is tetrahydrofuran-3-one substituted at positions 2 and 4 by hydroxy groups and at position 2 by a methyl group. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2R,4S)-2-methyl-2,4-dihydroxydihydrofuran-3-one
- (R)-DHMF
|
|---|
|
Chemical Formula: |
C5H8O4 |
|---|
| Average Molecular Weight: |
132.116 |
|---|
| Monoisotopic Molecular
Weight: |
132.04225 |
|---|
| InChI Key: |
CCVJVKWZZMMBHB-WVZVXSGGSA-N |
|---|
| InChI: | InChI=1S/C5H8O4/c1-5(8)4(7)3(6)2-9-5/h3,6,8H,2H2,1H3/t3-,5+/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2R,4S)-2,4-dihydroxy-2-methyldihydrofuran-3(2H)-one |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC1(O)(OCC(O)C(=O)1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as furanones. These are compounds containing a furan ring bearing a ketone group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Dihydrofurans |
|---|
| Sub Class | Furanones |
|---|
|
Direct Parent |
Furanones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 3-furanone
- Acyloin
- Oxolane
- Cyclic ketone
- Secondary alcohol
- Ketone
- Hemiacetal
- Oxacycle
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- a small molecule (CPD-10775)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | (S)-4,5-dihydroxypentan-2,3-dione → (2R,4S)-2-methyl-2,4-dihydroxydihydrofuran-3-one(2R,4S)-2-methyl-2,4-dihydroxydihydrofuran-3-one + Water → (2R,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM (2004)Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Molecular cell 15, Pubmed: 15350213
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|