|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120052 |
|---|
|
Identification |
|---|
| Name: |
6,7-dihydrobiopterin |
|---|
| Description: | Not Available |
|---|
|
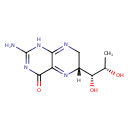
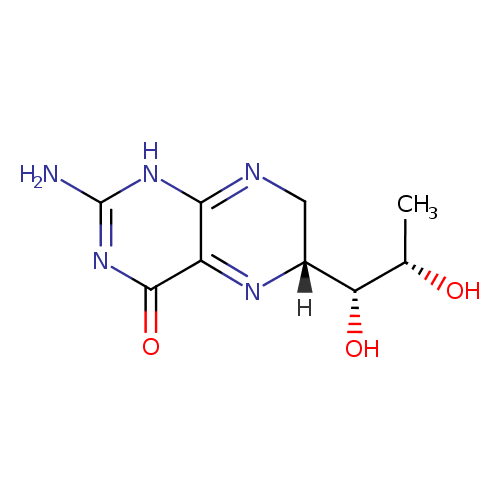
Structure |
|
|---|
| Synonyms: | - 6,7-dihydrobiopterin
- 6,7-Dihydrobiopterin
- 6,7-dihydrobiopterin
- Dihydrobiopterin
- q-dihydrobiopterin
- Quinoid-dihydrobiopterin
- quinoid-dihydrobiopterin
|
|---|
|
Chemical Formula: |
C9H13N5O3 |
|---|
| Average Molecular Weight: |
239.233 |
|---|
| Monoisotopic Molecular
Weight: |
239.10184 |
|---|
| InChI Key: |
ZHQJVZLJDXWFFX-BYAPIUGTSA-N |
|---|
| InChI: | InChI=1S/C9H13N5O3/c1-3(15)6(16)4-2-11-7-5(12-4)8(17)14-9(10)13-7/h3-4,6,15-16H,2H2,1H3,(H3,10,11,13,14,17)/t3-,4?,6-/m0/s1 |
|---|
| CAS
number: |
79647-29-3 |
|---|
| IUPAC Name: | 2-amino-6-(1,2-dihydroxypropyl)-7,8-dihydropteridin-4(6H)-one |
|---|
|
Traditional IUPAC Name: |
(6R)-2-amino-6-[(1R,2S)-1,2-dihydroxypropyl]-6,7-dihydro-1H-pteridin-4-one |
|---|
| SMILES: | CC(O)C(O)C2(N=C1(C(N=C(N)N=C1NC2)=O)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Pteridines and derivatives |
|---|
|
Direct Parent |
Biopterins and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Biopterin
- Aminopyrimidine
- Pyrimidone
- Primary aromatic amine
- Pyrimidine
- Imidolactam
- Vinylogous amide
- Heteroaromatic compound
- Secondary alcohol
- 1,2-diol
- Azacycle
- Amine
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
218 - 221 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 218 - 221 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Schallreuter KU, Wood JM, Pittelkow MR, Gutlich M, Lemke KR, Rodl W, Swanson NN, Hitzemann K, Ziegler I: Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin. Science. 1994 Mar 11;263(5152):1444-6. [8128228 ]
- Kim H, Roh H, Lee HJ, Chung SY, Choi SO, Lee KR, Han SB: Determination of phloroglucinol in human plasma by high-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 25;792(2):307-12. [12860038 ]
- Ismaili L, Refouvelet B, Xicluna A, Robert JF, Guillaume YC: Phloroglucinol: novel synthesis and role of the magnesium cation on its binding with human serum albumin (HSA) using a biochromatographic approach based on Langmuir isotherms. J Pharm Biomed Anal. 2003 Jul 14;32(3):549-53. [14565560 ]
- Jafri W, Yakoob J, Hussain S, Jafri N, Islam M: Phloroglucinol in irritable bowel syndrome. J Pak Med Assoc. 2006 Jan;56(1):5-8. [16454126 ]
- Fiset C, LeBel M: Influence of the menstrual cycle on the absorption and elimination of D-xylose. Clin Pharmacol Ther. 1990 Nov;48(5):529-36. [2225712 ]
- Armarego WL, Randles D, Taguchi H: Peroxidase catalysed aerobic degradation of 5,6,7,8-tetrahydrobiopterin at physiological pH. Eur J Biochem. 1983 Oct 3;135(3):393-403. [6617639 ]
- Davis MD, Kaufman S: Evidence for the formation of the 4a-carbinolamine during the tyrosine-dependent oxidation of tetrahydrobiopterin by rat liver phenylalanine hydroxylase. J Biol Chem. 1989 May 25;264(15):8585-96. [2722790 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|