|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120050 |
|---|
|
Identification |
|---|
| Name: |
cobalt-precorrin-7 |
|---|
| Description: | A precorrin carboxylic acid anion obtained by global deprotonation of the carboxy groups of cobalt-precorrin-7. |
|---|
|
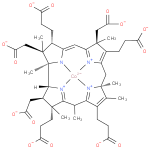
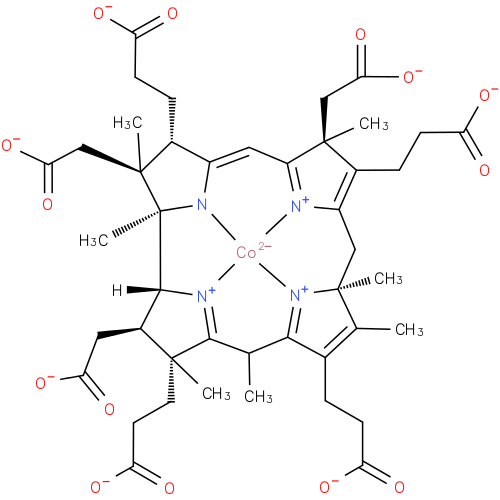
Structure |
|
|---|
| Synonyms: | - cobalt-precorrin-7
- cobalt-precorrin-7 heptaanion
|
|---|
|
Chemical Formula: |
C44H50N4O14CO |
|---|
| Average Molecular Weight: |
917.827 |
|---|
| Monoisotopic Molecular
Weight: |
924.3203 |
|---|
| InChI Key: |
HNBYEDSKGNPTKQ-LBSHSCIKSA-F |
|---|
| InChI: | InChI=1S/C44H58N4O14.Co/c1-21-37-23(8-11-30(49)50)22(2)43(6,48-37)18-28-24(9-12-31(51)52)41(4,19-35(59)60)29(45-28)17-27-25(10-13-32(53)54)42(5,20-36(61)62)44(7,47-27)39-26(16-34(57)58)40(3,38(21)46-39)15-14-33(55)56;/h17,21,25-26,39H,8-16,18-20H2,1-7H3,(H8,45,47,49,50,51,52,53,54,55,56,57,58,59,60,61,62);/q;+4/p-8/t21?,25-,26+,39-,40-,41+,42+,43?,44+;/m1./s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | Not Available |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC6(C7(C(C([CH]8(C2(N1([Co]4([N+]3(=C(C=C1C(CCC([O-])=O)C(CC(=O)[O-])(C)2)C(C(CCC(=O)[O-])=C3CC5(C(=C(CCC(=O)[O-])C(=[N+]45)6)C)C)(CC(=O)[O-])C))[N+]=78))C))CC(=O)[O-])(C)CCC(=O)[O-])) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as precorrins. These are intermediates formed by methylation at one or more of the four rings prior to the formation of the macrocyclic corrin ring. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Corrinoids |
|---|
|
Direct Parent |
Precorrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Precorrin
- Sesterterpenoid
- Metallotetrapyrrole skeleton
- Pyrrolidine
- Pyrroline
- Carboxylic acid salt
- Carboxylic acid derivative
- Carboxylic acid
- Metalloheterocycle
- Azacycle
- Organic transition metal salt
- Organic metal salt
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic salt
- Carbonyl group
- Organic anion
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -6 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
| Property | Value | Source |
|---|
| Molecular Weight | 959.824 g/mol | PubChem | | Hydrogen Bond Donor Count | 0 | PubChem | | Hydrogen Bond Acceptor Count | 20 | PubChem | | Rotatable Bond Count | 12 | PubChem | | Exact Mass | 959.24 g/mol | PubChem | | Monoisotopic Mass | 959.24 g/mol | PubChem | | Topological Polar Surface Area | 348 A^2 | PubChem | | Heavy Atom Count | 66 | PubChem | | Formal Charge | -8 | PubChem | | Complexity | 2290 | PubChem | | Isotope Atom Count | 0 | PubChem | | Defined Atom Stereocenter Count | 7 | PubChem | | Undefined Atom Stereocenter Count | 1 | PubChem | | Defined Bond Stereocenter Count | 2 | PubChem | | Undefined Bond Stereocenter Count | 0 | PubChem | | Covalently-Bonded Unit Count | 2 | PubChem |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- cob(II)yrinate a,c-diamide biosynthesis I (early cobalt insertion)PWY-7377
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Deery E, Schroeder S, Lawrence AD, Taylor SL, Seyedarabi A, Waterman J, Wilson KS, Brown D, Geeves MA, Howard MJ, Pickersgill RW, Warren MJ (2012)An enzyme-trap approach allows isolation of intermediates in cobalamin biosynthesis. Nature chemical biology 8, Pubmed: 23042036
- Roessner CA, Scott AI (2006)Fine-tuning our knowledge of the anaerobic route to cobalamin (vitamin B12). Journal of bacteriology 188, Pubmed: 16936030
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|