|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120047 |
|---|
|
Identification |
|---|
| Name: |
coproporphyrinogen I |
|---|
| Description: | The cyclic tetrapyrrole anion formed by loss of a proton from each of the four carboxy groups of coproporphyrinogen I. |
|---|
|
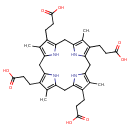
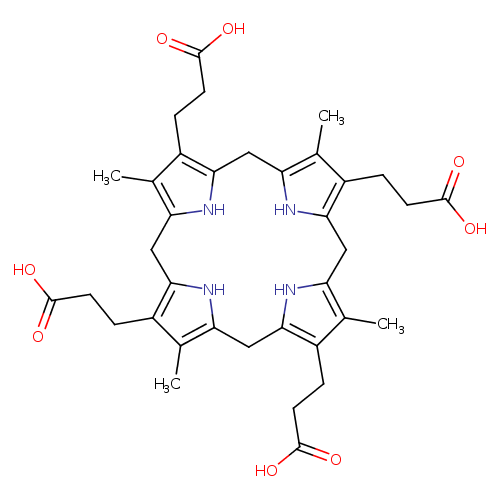
Structure |
|
|---|
| Synonyms: | - 3,3',3'',3'''-
 (3,8,13,18- (3,8,13,18- tetramethyl- tetramethyl- 5,10,15,20,22,24- 5,10,15,20,22,24- hexahydroporphyrin- hexahydroporphyrin- 2,7,12,17- 2,7,12,17- tetrayl)tetrapropanoate tetrayl)tetrapropanoate - 3,8,13,18-tetramethyl-5,10,15,20,22,24-hexahydroporphyrin-2,7,12,17-tetrapropionate
- coproporphyrinogen I
|
|---|
|
Chemical Formula: |
C36H40N4O8 |
|---|
| Average Molecular Weight: |
656.734 |
|---|
| Monoisotopic Molecular
Weight: |
660.3159 |
|---|
| InChI Key: |
WIUGGJKHYQIGNH-UHFFFAOYSA-J |
|---|
| InChI: | InChI=1S/C36H44N4O8/c1-17-21(5-9-33(41)42)29-14-26-19(3)23(7-11-35(45)46)31(39-26)16-28-20(4)24(8-12-36(47)48)32(40-28)15-27-18(2)22(6-10-34(43)44)30(38-27)13-25(17)37-29/h37-40H,5-16H2,1-4H3,(H,41,42)(H,43,44)(H,45,46)(H,47,48)/p-4 |
|---|
| CAS
number: |
31110-56-2 |
|---|
| IUPAC Name: | 3,8,13,18-tetramethyl-5,10,15,20,22,24-hexahydroporphyrin-2,7,12,17-tetrapropanoate |
|---|
|
Traditional IUPAC Name: |
coproporphyrinogen I |
|---|
| SMILES: | CC1(=C2(CC5(=C(CCC([O-])=O)C(C)=C(CC4(=C(CCC([O-])=O)C(C)=C(CC3(=C(CCC([O-])=O)C(C)=C(CC(=C(CCC([O-])=O)1)N2)N3))N4))N5))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Tetrapyrroles and derivatives |
|---|
|
Direct Parent |
Porphyrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Porphyrin
- Tetracarboxylic acid or derivatives
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Azacycle
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
171 - 174 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 171 - 174 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Ding Y, Lin B, Huie CW: Binding studies of porphyrins to human serum albumin using affinity capillary electrophoresis. Electrophoresis. 2001 Jul;22(11):2210-6. [11504054 ]
- Gorchein A, Guo R, Lim CK, Raimundo A, Pullon HW, Bellingham AJ: Porphyrins in urine, plasma, erythrocytes, bile and faeces in a case of congenital erythropoietic porphyria (Gunther's disease) treated with blood transfusion and iron chelation: lack of benefit from oral charcoal. Biomed Chromatogr. 1998 Nov-Dec;12(6):350-6. [9861496 ]
- Beukeveld GJ, In 't Veld G, Havinga R, Groen AK, Wolthers BG, Kuipers F: Relationship between biliary lipid and protoporphyrin secretion; potential role of mdr2 P-glycoprotein in hepatobiliary organic anion transport. J Hepatol. 1996 Mar;24(3):343-52. [8778203 ]
- Pinelli A, Mussini C, Bertolini B, Buratti M, Trivulzio S: Increased excretion of urine coproporphyrins during daunorubicin administration in patients affected by acute myelogenous leukemia. Pharmacol Res. 2003 Nov;48(5):515-8. [12967599 ]
- Sakai T, Niinuma Y, Yanagihara S, Ushio K: Liquid-chromatographic separation and determination of coproporphyrins I and III in urine. Clin Chem. 1983 Feb;29(2):350-3. [6821943 ]
- Cornford P: Transformation of porphobilinogen into porphyrins by preparations from human erythrocytes. Biochem J. 1964 Apr;91(1):64-73. [5833390 ]
- Pannier E, Viot G, Aubry MC, Grange G, Tantau J, Fallet-Bianco C, Muller F, Cabrol D: Congenital erythropoietic porphyria (Gunther's disease): two cases with very early prenatal manifestation and cystic hygroma. Prenat Diagn. 2003 Jan;23(1):25-30. [12533808 ]
- Mel'nikova YaI, Kravchuk ZI, Preygerzon VA, Martsev SP: Functional activation of antibodies on modification with Pd(II) coproporphyrin I N-Hydroxysuccinimide ester. Biochemistry (Mosc). 1997 Aug;62(8):924-7. [9360305 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|