|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120035 |
|---|
|
Identification |
|---|
| Name: |
isobutanol |
|---|
| Description: | An alkyl alcohol that is propan-1-ol substituted by a methyl group at position 2. |
|---|
|
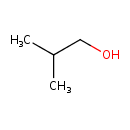
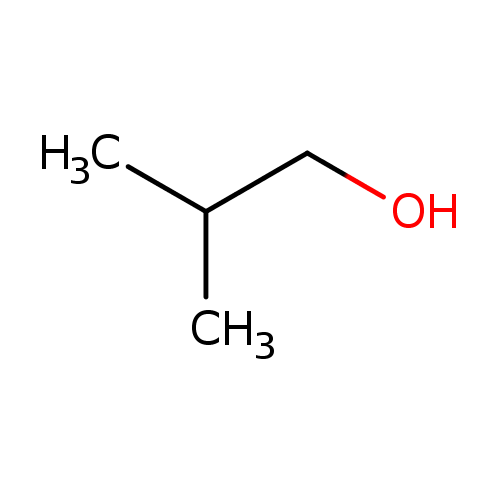
Structure |
|
|---|
| Synonyms: | - 1-hydroxymethylpropane
- 1-Hydroxymethylpropane
- 2-methyl-1-propanol
- 2-Methyl-1-propanol
- 2-methylpropanol
- i-Butanol
- i-Butyl alcohol
- IBA
- iso-butyl alcohol
- iso-C4H9OH
- isobutanol
- isobutyl alcohol
- Isobutylalkohol
- isopropylcarbinol
|
|---|
|
Chemical Formula: |
C4H10O |
|---|
| Average Molecular Weight: |
74.122 |
|---|
| Monoisotopic Molecular
Weight: |
74.073166 |
|---|
| InChI Key: |
ZXEKIIBDNHEJCQ-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H10O/c1-4(2)3-5/h4-5H,3H2,1-2H3 |
|---|
| CAS
number: |
78-83-1 |
|---|
| IUPAC Name: | 2-methylpropan-1-ol |
|---|
|
Traditional IUPAC Name: |
isobutanol |
|---|
| SMILES: | CC(C)CO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as primary alcohols. These are compounds comprising the primary alcohol functional group, with the general structure RCOH (R=alkyl, aryl). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
|
Class |
Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
|
Direct Parent |
Primary alcohols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydrocarbon derivative
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
- primary alcohol, alkyl alcohol (CHEBI:46645)
- Fatty alcohols (LMFA05000100)
- a small molecule (ISOBUTANOL)
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
-108 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | -108 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 85 mg/mL at 25 °C | Not Available | | LogP | 0.76 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Matsuda F, Ishii J, Kondo T, Ida K, Tezuka H, Kondo A (2013)Increased isobutanol production in Saccharomyces cerevisiae by eliminating competing pathways and resolving cofactor imbalance. Microbial cell factories 12, Pubmed: 24305546
- Desai SH, Rabinovitch-Deere CA, Tashiro Y, Atsumi S (2014)Isobutanol production from cellobiose in Escherichia coli. Applied microbiology and biotechnology 98, Pubmed: 24430208
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|