|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120028 |
|---|
|
Identification |
|---|
| Name: |
4-coumaryl-CoA |
|---|
| Description: | The S-(4-coumaroyl) derivative of coenzyme A. |
|---|
|
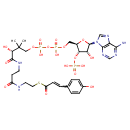
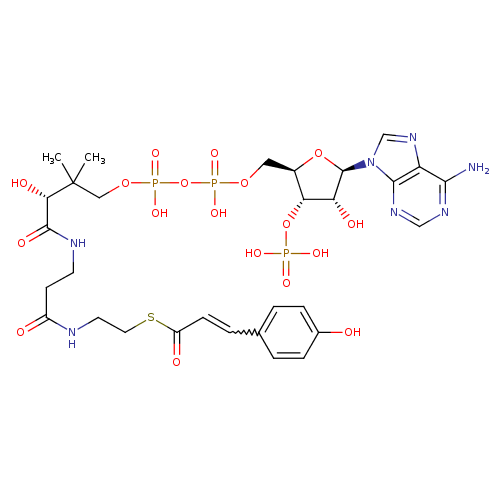
Structure |
|
|---|
| Synonyms: | - 4-Coumaroyl-CoA
- 4-Coumaroyl-coa
- 4-Coumaroyl-CoA
- 4-Coumaroyl-coenzyme A
- 4-Hydroxycinnamoyl-CoA
|
|---|
|
Chemical Formula: |
C30H38N7O18P3S |
|---|
| Average Molecular Weight: |
909.648 |
|---|
| Monoisotopic Molecular
Weight: |
913.152 |
|---|
| InChI Key: |
DMZOKBALNZWDKI-MATMFAIHSA-J |
|---|
| InChI: | InChI=1S/C30H42N7O18P3S/c1-30(2,25(42)28(43)33-10-9-20(39)32-11-12-59-21(40)8-5-17-3-6-18(38)7-4-17)14-52-58(49,50)55-57(47,48)51-13-19-24(54-56(44,45)46)23(41)29(53-19)37-16-36-22-26(31)34-15-35-27(22)37/h3-8,15-16,19,23-25,29,38,41-42H,9-14H2,1-2H3,(H,32,39)(H,33,43)(H,47,48)(H,49,50)(H2,31,34,35)(H2,44,45,46)/p-4/b8-5+/t19-,23-,24-,25+,29-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3'- phosphoadenosine 5'- phosphoadenosine 5'- {3- {3- [(3R)- [(3R)- 3- 3- hydroxy- hydroxy- 4- 4- ({3- ({3- [(2- [(2- {[3- {[3- (4- (4- hydroxyphenyl)prop- hydroxyphenyl)prop- 2- 2- enoyl]sulfanyl}ethyl)amino]- enoyl]sulfanyl}ethyl)amino]- 3- 3- oxopropyl}amino)- oxopropyl}amino)- 2,2- 2,2- dimethyl- dimethyl- 4- 4- oxobutyl] dihydrogen diphosphate} oxobutyl] dihydrogen diphosphate} |
|---|
|
Traditional IUPAC Name: |
4-coumaroyl-coa |
|---|
| SMILES: | CC(C)(C(O)C(=O)NCCC(=O)NCCSC(=O)C=CC1(C=CC(O)=CC=1))COP(=O)(OP(=O)(OCC2(C(OP([O-])(=O)[O-])C(O)C(O2)N4(C3(=C(C(N)=NC=N3)N=C4))))[O-])[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
|
Direct Parent |
Acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside diphosphate
- Purine ribonucleoside 3',5'-bisphosphate
- Hydroxycinnamic acid or derivatives
- Coumaric acid or derivatives
- Cinnamic acid or derivatives
- N-glycosyl compound
- Glycosyl compound
- Organic pyrophosphate
- Monosaccharide phosphate
- 6-aminopurine
- Purine
- Phenylpropene
- Imidazopyrimidine
- Styrene
- Monoalkyl phosphate
- Phenol
- Aminopyrimidine
- Imidolactam
- Benzenoid
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- Monosaccharide
- Saccharide
- Monocyclic benzene moiety
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Thiocarboxylic acid ester
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Sulfenyl compound
- Thioether
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboximidic acid derivative
- Carboximidic acid
- Hydrocarbon derivative
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, Thorleifsson SG, Agren R, Bolling C, Bordel S, Chavali AK, Dobson P, Dunn WB, Endler L, Hala D, Hucka M, Hull D, Jameson D, Jamshidi N, Jonsson JJ, Juty N, Keating S, Nookaew I, Le Novere N, Malys N, Mazein A, Papin JA, Price ND, Selkov E Sr, Sigurdsson MI, Simeonidis E, Sonnenschein N, Smallbone K, Sorokin A, van Beek JH, Weichart D, Goryanin I, Nielsen J, Westerhoff HV, Kell DB, Mendes P, Palsson BO: A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 Mar 3. doi: 10.1038/nbt.2488. [23455439 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|